Progress in agonist therapy for substance use disorders: Lessons learned from methadone and buprenorphine
- PMID: 31009632
- PMCID: PMC6745247
- DOI: 10.1016/j.neuropharm.2019.04.015
Progress in agonist therapy for substance use disorders: Lessons learned from methadone and buprenorphine
Abstract
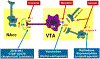
Substance use disorders (SUD) are serious public health problems worldwide. Although significant progress has been made in understanding the neurobiology of drug reward and the transition to addiction, effective pharmacotherapies for SUD remain limited and a majority of drug users relapse even after a period of treatment. The United States Food and Drug Administration (FDA) has approved several medications for opioid, nicotine, and alcohol use disorders, whereas none are approved for the treatment of cocaine or other psychostimulant use disorders. The medications approved by the FDA for the treatment of SUD can be divided into two major classes - agonist replacement therapies, such as methadone and buprenorphine for opioid use disorders (OUD), nicotine replacement therapy (NRT) and varenicline for nicotine use disorders (NUD), and antagonist therapies, such as naloxone for opioid overdose and naltrexone for promoting abstinence. In the present review, we primarily focus on the pharmacological rationale of agonist replacement strategies in treatment of opioid dependence, and the potential translation of this rationale to new therapies for cocaine use disorders. We begin by describing the neural mechanisms underlying opioid reward, followed by preclinical and clinical findings supporting the utility of agonist therapies in the treatment of OUD. We then discuss recent progress of agonist therapies for cocaine use disorders based on lessons learned from methadone and buprenorphine. We contend that future studies should identify agonist pharmacotherapies that can facilitate abstinence in patients who are motivated to quit their illicit drug use. Focusing on those that are able to achieve abstinence from cocaine will provide a platform to broaden the effectiveness of medication and psychosocial treatment strategies for this underserved population. This article is part of the Special Issue entitled 'New Vistas in Opioid Pharmacology'.
Keywords: Addiction; Agonist replacement therapy; Atypical dopamine uptake inhibitor; Cocaine; Dopamine transporter; Methadone; Opioids; Substance use disorders.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Mixed κ/μ partial opioid agonists as potential treatments for cocaine dependence.Adv Pharmacol. 2014;69:387-418. doi: 10.1016/B978-0-12-420118-7.00010-X. Adv Pharmacol. 2014. PMID: 24484983 Review.
-
Maintenance medication for opiate addiction: the foundation of recovery.J Addict Dis. 2012;31(3):207-25. doi: 10.1080/10550887.2012.694598. J Addict Dis. 2012. PMID: 22873183 Free PMC article. Review.
-
Medication Treatment of Opioid Use Disorder.Biol Psychiatry. 2020 Jan 1;87(1):82-88. doi: 10.1016/j.biopsych.2019.06.020. Epub 2019 Jul 2. Biol Psychiatry. 2020. PMID: 31420089 Review.
-
Desipramine in opioid-dependent cocaine abusers maintained on buprenorphine vs methadone.Arch Gen Psychiatry. 1999 Sep;56(9):812-20. doi: 10.1001/archpsyc.56.9.812. Arch Gen Psychiatry. 1999. PMID: 12884887 Clinical Trial.
-
Dopamine D3 receptor-based medication development for the treatment of opioid use disorder: Rationale, progress, and challenges.Neurosci Biobehav Rev. 2020 Jul;114:38-52. doi: 10.1016/j.neubiorev.2020.04.024. Epub 2020 May 3. Neurosci Biobehav Rev. 2020. PMID: 32376243 Free PMC article. Review.
Cited by
-
The exploration of optimized protocol for repetitive transcranial magnetic stimulation in the treatment of methamphetamine use disorder: A randomized sham-controlled study.EBioMedicine. 2020 Oct;60:103027. doi: 10.1016/j.ebiom.2020.103027. Epub 2020 Sep 25. EBioMedicine. 2020. PMID: 32980696 Free PMC article. Clinical Trial.
-
A mechanistic overview of approaches for the treatment of psychostimulant dependence.Front Pharmacol. 2022 Sep 8;13:854176. doi: 10.3389/fphar.2022.854176. eCollection 2022. Front Pharmacol. 2022. PMID: 36160447 Free PMC article. Review.
-
Is Cocaine Protonated When it Binds to the Dopamine Transporter?JACS Au. 2025 Feb 20;5(3):1157-1172. doi: 10.1021/jacsau.4c00952. eCollection 2025 Mar 24. JACS Au. 2025. PMID: 40151268 Free PMC article.
-
Potential of Cannabinoid Receptor Ligands as Treatment for Substance Use Disorders.CNS Drugs. 2019 Oct;33(10):1001-1030. doi: 10.1007/s40263-019-00664-w. CNS Drugs. 2019. PMID: 31549358 Free PMC article. Review.
-
Series of (([1,1'-Biphenyl]-2-yl)methyl)sulfinylalkyl Alicyclic Amines as Novel and High Affinity Atypical Dopamine Transporter Inhibitors with Reduced hERG Activity.ACS Pharmacol Transl Sci. 2024 Jan 5;7(2):515-532. doi: 10.1021/acsptsci.3c00322. eCollection 2024 Feb 9. ACS Pharmacol Transl Sci. 2024. PMID: 38357284 Free PMC article.
References
-
- Agoston GE, Wu JH, Izenwasser S, George C, Katz J, et al. 1997. Novel N-substituted 3 alpha-[bis(4’-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem 40: 4329–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

