Spatial T-maze identifies cognitive deficits in piglets 1 month after hypoxia-ischemia in a model of hippocampal pyramidal neuron loss and interneuron attrition
- PMID: 31009645
- PMCID: PMC6545140
- DOI: 10.1016/j.bbr.2019.111921
Spatial T-maze identifies cognitive deficits in piglets 1 month after hypoxia-ischemia in a model of hippocampal pyramidal neuron loss and interneuron attrition
Abstract
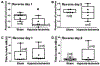
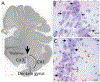
Neonatal brain injury from hypoxia-ischemia (HI) causes major morbidity. Piglet HI is an established method for testing neuroprotective treatments in large, gyrencephalic brain. Though many neurobehavior tests exist for rodents, such tests and their associations with neuropathologic injury remain underdeveloped and underutilized in large, neonatal HI animal models. We examined whether spatial T-maze and inclined beam tests distinguish cognitive and motor differences between HI and sham piglets and correlate with neuropathologic injury. Neonatal piglets were randomized to whole-body HI or sham procedure, and they began T-maze and inclined beam testing 17 days later. HI piglets had more incorrect T-maze turns than did shams. Beam walking time did not differ between groups. Neuropathologic evaluations at 33 days validated the injury with putamen neuron loss after HI to below that of sham procedure. HI decreased the numbers of CA3 pyramidal neurons but not CA1 pyramidal neurons or dentate gyrus granule neurons. Though the number of hippocampal parvalbumin-positive interneurons did not differ between groups, HI reduced the number of CA1 interneuron dendrites. Piglets with more incorrect turns had greater CA3 neuron loss, and piglets that took longer in the maze had fewer CA3 interneurons. The number of putamen neurons was unrelated to T-maze or beam performance. We conclude that neonatal HI causes hippocampal CA3 neuron loss, CA1 interneuron dendritic attrition, and putamen neuron loss at 1-month recovery. The spatial T-maze identifies learning and memory deficits that are related to loss of CA3 pyramidal neurons and fewer parvalbumin-positive interneurons independent of putamen injury.
Keywords: Cognition; Hippocampus; Hypoxia; Learning; Newborn; putamen.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures

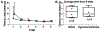




Similar articles
-
Dendritic development of hippocampal CA1 pyramidal cells in a neonatal hypoxia-ischemia injury model.J Neurosci Res. 2013 Sep;91(9):1165-73. doi: 10.1002/jnr.23247. Epub 2013 May 20. J Neurosci Res. 2013. PMID: 23686818
-
Delayed injury of hippocampal interneurons after neonatal hypoxia-ischemia and therapeutic hypothermia in a murine model.Hippocampus. 2018 Aug;28(8):617-630. doi: 10.1002/hipo.22965. Hippocampus. 2018. PMID: 29781223 Free PMC article.
-
Effects of age, experience and inter-alpha inhibitor proteins on working memory and neuronal plasticity after neonatal hypoxia-ischemia.Behav Brain Res. 2016 Apr 1;302:88-99. doi: 10.1016/j.bbr.2016.01.016. Epub 2016 Jan 8. Behav Brain Res. 2016. PMID: 26778784 Free PMC article.
-
Interneurons in rat hippocampus after cerebral ischemia. Morphometric, functional, and therapeutic investigations.Acta Neurol Scand Suppl. 1993;150:1-32. Acta Neurol Scand Suppl. 1993. PMID: 7907456 Review.
-
Selective vulnerability of hippocampal CA1 and CA3 pyramidal cells: What are possible pathomechanisms and should more attention be paid to the CA3 region in future studies?J Neurosci Res. 2024 Jan;102(1):e25276. doi: 10.1002/jnr.25276. J Neurosci Res. 2024. PMID: 38284845 Review.
Cited by
-
Breathing Signature as Vitality Score Index Created by Exercises of Qigong: Implications of Artificial Intelligence Tools Used in Traditional Chinese Medicine.J Funct Morphol Kinesiol. 2019 Dec;4(4):71. doi: 10.3390/jfmk4040071. Epub 2019 Dec 3. J Funct Morphol Kinesiol. 2019. PMID: 31853512 Free PMC article.
-
Hypothermic Protection in Neocortex Is Topographic and Laminar, Seizure Unmitigating, and Partially Rescues Neurons Depleted of RNA Splicing Protein Rbfox3/NeuN in Neonatal Hypoxic-Ischemic Male Piglets.Cells. 2023 Oct 15;12(20):2454. doi: 10.3390/cells12202454. Cells. 2023. PMID: 37887298 Free PMC article.
-
Hypothermia Shifts Neurodegeneration Phenotype in Neonatal Human Hypoxic-Ischemic Encephalopathy but Not in Related Piglet Models: Possible Relationship to Toxic Conformer and Intrinsically Disordered Prion-like Protein Accumulation.Cells. 2025 Apr 12;14(8):586. doi: 10.3390/cells14080586. Cells. 2025. PMID: 40277911 Free PMC article.
-
A novel porcine model of CLN3 Batten disease recapitulates clinical phenotypes.Dis Model Mech. 2023 Aug 1;16(8):dmm050038. doi: 10.1242/dmm.050038. Epub 2023 Aug 7. Dis Model Mech. 2023. PMID: 37305926 Free PMC article.
-
Milk fat globule membrane promotes brain development in piglets by enhancing the connection of white matter fiber trace.Front Nutr. 2023 Nov 23;10:1248809. doi: 10.3389/fnut.2023.1248809. eCollection 2023. Front Nutr. 2023. PMID: 38075212 Free PMC article.
References
-
- Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, Petrie Huitema CM, Ehrenkranz RA, Tyson JE, Das A, Hammond J, Peralta-Carcelen M, Evans PW, Heyne RJ, Wilson-Costello DE, Vaucher YE, Bauer CR, Dusick AM, Adams-Chapman I, Goldstein RF, Guillet R, Papile LA, Higgins RD, Eunice Kennedy Shriver NICHD Neonatal Research Network. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med, 2012;366:2085–2092 doi: 10.1056/NEJMoa1112066. - DOI - PMC - PubMed
-
- Burton VJ, Gerner G, Cristofalo E, Chung SE, Jennings JM, Parkinson C, Koehler RC, Chavez-Valdez R, Johnston MV, Northington FJ, Lee JK. A pilot cohort study of cerebral autoregulation and 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia. BMC Neurol, 2015;15:209-015-0464-4 doi: 10.1186/s12883-015-0464-4 [doi]. - DOI - PMC - PubMed
-
- Ezzati M, Kawano G, Rocha-Ferreira E, Alonso-Alconada D, Hassell JK, Broad KD, Fierens I, Fleiss B, Bainbridge A, Price DL, Kaynezhad P, Anderson B, Hristova M, Tachtsidis I, Golay X, Gressens P, Sanders RD, Robertson NJ. Dexmedetomidine Combined with Therapeutic Hypothermia Is Associated with Cardiovascular Instability and Neurotoxicity in a Piglet Model of Perinatal Asphyxia. Dev Neurosci, 2017;39:156–170 doi: 10.1159/000458438 [doi]. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

