Research progress in the biological activities of 3,4,5-trimethoxycinnamic acid (TMCA) derivatives
- PMID: 31009908
- PMCID: PMC7115657
- DOI: 10.1016/j.ejmech.2019.04.009
Research progress in the biological activities of 3,4,5-trimethoxycinnamic acid (TMCA) derivatives
Abstract
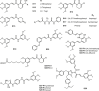
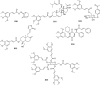
TMCA (3,4,5-trimethoxycinnamic acid) ester and amide are privileged structural scaffolds in drug discovery which are widely distributed in natural products and consequently produced diverse therapeutically relevant pharmacological functions. Owing to the potential of TMCA ester and amide analogues as therapeutic agents, researches on chemical syntheses and modifications have been carried out to drug-like candidates with broad range of medicinal properties such as antitumor, antiviral, CNS (central nervous system) agents, antimicrobial, anti-inflammatory and hematologic agents for a long time. At the same time, SAR (structure-activity relationship) studies have draw greater attention among medicinal chemists, and many of the lead compounds were derived for various disease targets. However, there is an urgent need for the medicinal chemists to further exploit the precursor in developing chemical entities with promising bioactivity and druggability. This review concisely summarizes the synthesis and biological activity for TMCA ester and amide analogues. It also comprehensively reveals the relationship of significant biological activities along with SAR studies.
Keywords: 3,4,5-Trimethoxycinnamic acid; Antimicrobial agents; Antitumor agents; Antiviral agents; CNS agnets; Hematologic agents; SAR; TMCA derivatives; anti-inflammatory agents.
Copyright © 2019 Elsevier Masson SAS. All rights reserved.
Figures
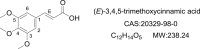








Similar articles
-
Pyrazolone structural motif in medicinal chemistry: Retrospect and prospect.Eur J Med Chem. 2020 Jan 15;186:111893. doi: 10.1016/j.ejmech.2019.111893. Epub 2019 Nov 16. Eur J Med Chem. 2020. PMID: 31761383 Free PMC article. Review.
-
Triazine as a promising scaffold for its versatile biological behavior.Eur J Med Chem. 2015 Sep 18;102:39-57. doi: 10.1016/j.ejmech.2015.07.037. Epub 2015 Jul 21. Eur J Med Chem. 2015. PMID: 26241876 Review.
-
Research progress in biological activities of succinimide derivatives.Bioorg Chem. 2021 Mar;108:104557. doi: 10.1016/j.bioorg.2020.104557. Epub 2020 Dec 15. Bioorg Chem. 2021. PMID: 33376010 Review.
-
Research progress in biological activities of isochroman derivatives.Eur J Med Chem. 2021 Jan 15;210:113073. doi: 10.1016/j.ejmech.2020.113073. Epub 2020 Dec 2. Eur J Med Chem. 2021. PMID: 33310287 Review.
-
Key Updates on the Chemistry and Biological Roles of Thiazine Scaffold: A Review.Mini Rev Med Chem. 2018;18(17):1452-1478. doi: 10.2174/1389557518666180416150552. Mini Rev Med Chem. 2018. PMID: 29663882 Review.
Cited by
-
Pravastatin promotes type 2 diabetes vascular calcification through activating intestinal Bacteroides fragilis to induce macrophage M1 polarization.J Diabetes. 2024 Jun;16(6):e13514. doi: 10.1111/1753-0407.13514. Epub 2023 Dec 19. J Diabetes. 2024. PMID: 38112268 Free PMC article.
-
Study of the Antibacterial Activity of Rich Polyphenolic Extracts Obtained from Cytisus scoparius against Foodborne Pathogens.Antibiotics (Basel). 2023 Nov 20;12(11):1645. doi: 10.3390/antibiotics12111645. Antibiotics (Basel). 2023. PMID: 37998847 Free PMC article.
-
The Efficacy of Cholesterol-Based Carriers in Drug Delivery.Molecules. 2020 Sep 22;25(18):4330. doi: 10.3390/molecules25184330. Molecules. 2020. PMID: 32971733 Free PMC article. Review.
-
Pyrazolone structural motif in medicinal chemistry: Retrospect and prospect.Eur J Med Chem. 2020 Jan 15;186:111893. doi: 10.1016/j.ejmech.2019.111893. Epub 2019 Nov 16. Eur J Med Chem. 2020. PMID: 31761383 Free PMC article. Review.
-
Challenges Faced with Small Molecular Modulators of Potassium Current Channel Isoform Kv1.5.Biomolecules. 2019 Dec 19;10(1):10. doi: 10.3390/biom10010010. Biomolecules. 2019. PMID: 31861703 Free PMC article. Review.
References
-
- Duarte F.S., Marder M., Hoeller A.A., Duzzioni M., Mendes B.G., Pizzolatti M.G., De Lima T.C.M. Anticonvulsant and anxiolytic-like effects of compounds isolated from Polygala sabulosa (Polygalaceae) in rodents: in vitro and in vivo interactions with benzodiazepine binding sites. Psychopharmacology. 2008;197:351–360. - PubMed
-
- Klein L.C., Jr., de Andrade S.F., Cechinel Filho V. A pharmacognostic approach to the Polygala genus: phytochemical and pharmacological aspects. Chem. Biodivers. 2012;9:181–209. - PubMed
-
- Lee C.I., Han J.Y., Hong J.T., Oh K.W. 3,4,5-Trimethoxycinnamic acid (TMCA), one of the constituents of Polygalae Radix enhances pentobarbital-induced sleeping behaviors via GABAAergic systems in mice. Arch Pharm. Res. (Seoul) 2013;36:1244–1251. - PubMed
-
- Chen C.Y., Wei X.D., Chen C.R. 3,4,5-Trimethoxycinnamic acid, one of the constituents of Polygalae Radix exerts anti-seizure effects by modulating GABAAergic systems in mice. J. Pharmacol. Sci. 2016;131:1–5. - PubMed
-
- Jung J.C., Min D., Kim H., Jang S., Lee Y., Park W.K., Khan I.A., Moon H.I., Jung M., Oh S. Design, synthesis, and biological evaluation of 3,4,5-trimethoxyphenyl acrylamides as antinarcotic agents. J. Enzym. Inhib. Med. Chem. 2010;25:38–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

