PHB is Produced from Glycogen Turn-over during Nitrogen Starvation in Synechocystis sp. PCC 6803
- PMID: 31010017
- PMCID: PMC6514691
- DOI: 10.3390/ijms20081942
PHB is Produced from Glycogen Turn-over during Nitrogen Starvation in Synechocystis sp. PCC 6803
Abstract
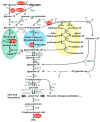
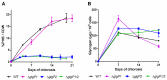
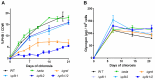
Polyhydroxybutyrate (PHB) is a polymer of great interest as a substitute for conventional plastics, which are becoming an enormous environmental problem. PHB can be produced directly from CO2 in photoautotrophic cyanobacteria. The model cyanobacterium Synechocystis sp. PCC 6803 produces PHB under conditions of nitrogen starvation. However, it is so far unclear which metabolic pathways provide the precursor molecules for PHB synthesis during nitrogen starvation. In this study, we investigated if PHB could be derived from the main intracellular carbon pool, glycogen. A mutant of the major glycogen phosphorylase, GlgP2 (slr1367 product), was almost completely impaired in PHB synthesis. Conversely, in the absence of glycogen synthase GlgA1 (sll0945 product), cells not only produced less PHB, but were also impaired in acclimation to nitrogen depletion. To analyze the role of the various carbon catabolic pathways (EMP, ED and OPP pathways) for PHB production, mutants of key enzymes of these pathways were analyzed, showing different impact on PHB synthesis. Together, this study clearly indicates that PHB in glycogen-producing Synechocystis sp. PCC 6803 cells is produced from this carbon-pool during nitrogen starvation periods. This knowledge can be used for metabolic engineering to get closer to the overall goal of a sustainable, carbon-neutral bioplastic production.
Keywords: PHB; Synechocystis; bioplastic; cyanobacteria; glycogen; metabolic engineering; sustainable.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Requirement of the nitrogen starvation-induced protein Sll0783 for polyhydroxybutyrate accumulation in Synechocystis sp. strain PCC 6803.Appl Environ Microbiol. 2010 Sep;76(18):6101-7. doi: 10.1128/AEM.00484-10. Epub 2010 Jul 30. Appl Environ Microbiol. 2010. PMID: 20675451 Free PMC article.
-
One day of nitrogen starvation reveals the effect of sigE and rre37 overexpression on the expression of genes related to carbon and nitrogen metabolism in Synechocystis sp. PCC 6803.J Biosci Bioeng. 2015 Aug;120(2):128-34. doi: 10.1016/j.jbiosc.2014.12.020. Epub 2015 Jan 29. J Biosci Bioeng. 2015. PMID: 25641577
-
Novel quantitative insights into carbon sources for synthesis of poly hydroxybutyrate in Synechocystis PCC 6803.Photosynth Res. 2018 Jun;136(3):303-314. doi: 10.1007/s11120-017-0464-x. Epub 2017 Nov 9. Photosynth Res. 2018. PMID: 29124651
-
Production of Bioplastic Compounds by Genetically Manipulated and Metabolic Engineered Cyanobacteria.Adv Exp Med Biol. 2018;1080:155-169. doi: 10.1007/978-981-13-0854-3_7. Adv Exp Med Biol. 2018. PMID: 30091095 Review.
-
Polyhydroxybutyrate: A Useful Product of Chlorotic Cyanobacteria.Microb Physiol. 2021;31(2):67-77. doi: 10.1159/000515617. Epub 2021 May 12. Microb Physiol. 2021. PMID: 33979794 Review.
Cited by
-
Deciphering the genetic landscape of enhanced poly-3-hydroxybutyrate production in Synechocystis sp. B12.Biotechnol Biofuels Bioprod. 2024 Jul 16;17(1):101. doi: 10.1186/s13068-024-02548-8. Biotechnol Biofuels Bioprod. 2024. PMID: 39014484 Free PMC article.
-
Screening novel genes by a comprehensive strategy to construct multiple stress-tolerant industrial Saccharomyces cerevisiae with prominent bioethanol production.Biotechnol Biofuels Bioprod. 2022 Jan 21;15(1):11. doi: 10.1186/s13068-022-02109-x. Biotechnol Biofuels Bioprod. 2022. PMID: 35418148 Free PMC article.
-
Enhanced productivity of extracellular free fatty acids by gene disruptions of acyl-ACP synthetase and S-layer protein in Synechocystis sp. PCC 6803.Biotechnol Biofuels Bioprod. 2022 Sep 24;15(1):99. doi: 10.1186/s13068-022-02197-9. Biotechnol Biofuels Bioprod. 2022. PMID: 36153604 Free PMC article.
-
Heavy Metal Stress Alters the Response of the Unicellular Cyanobacterium Synechococcus elongatus PCC 7942 to Nitrogen Starvation.Life (Basel). 2020 Nov 7;10(11):275. doi: 10.3390/life10110275. Life (Basel). 2020. PMID: 33171751 Free PMC article.
-
Polyhydroxybutyrate-producing cyanobacteria from lampenflora: The case study of the "Stiffe" caves in Italy.Front Microbiol. 2022 Jul 28;13:933398. doi: 10.3389/fmicb.2022.933398. eCollection 2022. Front Microbiol. 2022. PMID: 35966678 Free PMC article.
References
-
- Vitousek P.M., Howarth R.W. Nitrogen Limitation on Land and in the Sea—How Can. It Occur. Biogeochemistry. 1991;13:87–115. doi: 10.1007/BF00002772. - DOI
-
- Kaneko T., Tanaka A., Sato S., Kotani H., Sazuka T., Miyajima N., Sugiura M., Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. I. Sequence features in the 1 Mb region from map positions 64% to 92% of the genome. DNA Res. 1995;2:153–166. doi: 10.1093/dnares/2.4.153. - DOI - PubMed
-
- Osanai T., Oikawa A., Shirai T., Kuwahara A., Iijima H., Tanaka K., Ikeuchi M., Kondo A., Saito K., Hirai M.Y. Capillary electrophoresis-mass spectrometry reveals the distribution of carbon metabolites during nitrogen starvation in Synechocystis sp. PCC 6803. Environ. Microbiol. 2014;16:512–524. doi: 10.1111/1462-2920.12170. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

