Effect of Electrospun Fiber Mat Thickness and Support Method on Cell Morphology
- PMID: 31010029
- PMCID: PMC6523829
- DOI: 10.3390/nano9040644
Effect of Electrospun Fiber Mat Thickness and Support Method on Cell Morphology
Abstract
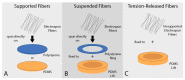
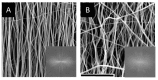
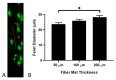
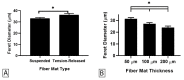
Electrospun fiber mats (EFMs) are highly versatile biomaterials used in a myriad of biomedical applications. Whereas some facets of EFMs are well studied and can be highly tuned (e.g., pore size, fiber diameter, etc.), other features are under characterized. For example, although substrate mechanics have been explored by several groups, most studies rely on Young's modulus alone as a characterization variable. The influence of fiber mat thickness and the effect of supports are variables that are often not considered when evaluating cell-mechanical response. To assay the role of these features in EFM scaffold design and to improve understanding of scaffold mechanical properties, we designed EFM scaffolds with varying thickness (50-200 µm) and supporting methodologies. EFM scaffolds were comprised of polycaprolactone and were either electrospun directly onto a support, suspended across an annulus (3 or 10 mm inner diameter), or "tension-released" and then suspended across an annulus. Then, single cell spreading (i.e., Feret diameter) was measured in the presence of these different features. Cells were sensitive to EFM thickness and suspended gap diameter. Overall, cell spreading was greatest for 50 µm thick EFMs suspended over a 3 mm gap, which was the smallest thickness and gap investigated. These results are counterintuitive to conventional understanding in mechanobiology, which suggests that stiffer materials, such as thicker, supported EFMs, should elicit greater cell polarization. Additional experiments with 50 µm thick EFMs on polystyrene and polydimethylsiloxane (PDMS) supports demonstrated that cells can "feel" the support underlying the EFM if it is rigid, similar to previous results in hydrogels. These results also suggest that EFM curvature may play a role in cell response, separate from Young's modulus, possibly because of internal tension generated. These parameters are not often considered in EFM design and could improve scaffold performance and ultimately patient outcomes.
Keywords: biomaterials; electrospun fiber mats; finite element modeling; glioblastoma; mechanobiology.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Cell attachment to hydrogel-electrospun fiber mat composite materials.J Funct Biomater. 2012 Jul 27;3(3):497-513. doi: 10.3390/jfb3030497. J Funct Biomater. 2012. PMID: 24955629 Free PMC article.
-
Effects of hydrophobicity and mat thickness on release from hydrogel-electrospun fiber mat composites.J Biomater Sci Polym Ed. 2013;24(17):2018-30. doi: 10.1080/09205063.2013.822246. Epub 2013 Aug 1. J Biomater Sci Polym Ed. 2013. PMID: 23905840
-
Elastic moduli of electrospun mats: Importance of fiber curvature and specimen dimensions.J Mech Behav Biomed Mater. 2017 Aug;72:6-13. doi: 10.1016/j.jmbbm.2017.04.013. Epub 2017 Apr 12. J Mech Behav Biomed Mater. 2017. PMID: 28433000
-
Biomedical application and controlled drug release of electrospun fibrous materials.Mater Sci Eng C Mater Biol Appl. 2018 Sep 1;90:750-763. doi: 10.1016/j.msec.2018.05.007. Epub 2018 May 4. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 29853146 Review.
-
Cell-matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: Implications for scaffold design and performance.Acta Biomater. 2017 Mar 1;50:41-55. doi: 10.1016/j.actbio.2016.12.034. Epub 2016 Dec 21. Acta Biomater. 2017. PMID: 28011142 Review.
Cited by
-
Effects of Fiber Density and Strain Rate on the Mechanical Properties of Electrospun Polycaprolactone Nanofiber Mats.Front Chem. 2020 Jul 21;8:610. doi: 10.3389/fchem.2020.00610. eCollection 2020. Front Chem. 2020. PMID: 32793555 Free PMC article.
-
Electrospun Poly(ethylene Terephthalate)/Silk Fibroin Composite for Filtration Application.Polymers (Basel). 2021 Jul 29;13(15):2499. doi: 10.3390/polym13152499. Polymers (Basel). 2021. PMID: 34372102 Free PMC article.
-
A State-of-the-Art of Functional Scaffolds for 3D Nervous Tissue Regeneration.Front Bioeng Biotechnol. 2021 Mar 18;9:639765. doi: 10.3389/fbioe.2021.639765. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33816451 Free PMC article. Review.
References
-
- Yun K.M., Hogan C.J., Jr., Matsubayashi Y., Kawabe M., Iskandar F., Okuyama K. Nanoparticle filtration by electrospun polymer fibers. Chem. Eng. Sci. 2007;62:4751–4759. doi: 10.1016/j.ces.2007.06.007. - DOI
-
- Deitzel J.M., Kleinmeyer J., Harris D.E.A., Polymer N.C.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer. 2001;42:261–272. doi: 10.1016/S0032-3861(00)00250-0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

