Fabrication and Applications of Solid-State Nanopores
- PMID: 31010038
- PMCID: PMC6515193
- DOI: 10.3390/s19081886
Fabrication and Applications of Solid-State Nanopores
Abstract
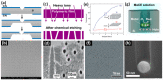
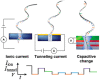
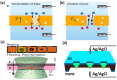
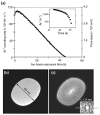
Nanopores fabricated from synthetic materials (solid-state nanopores), platforms for characterizing biological molecules, have been widely studied among researchers. Compared with biological nanopores, solid-state nanopores are mechanically robust and durable with a tunable pore size and geometry. Solid-state nanopores with sizes as small as 1.3 nm have been fabricated in various films using engraving techniques, such as focused ion beam (FIB) and focused electron beam (FEB) drilling methods. With the demand of massively parallel sensing, many scalable fabrication strategies have been proposed. In this review, typical fabrication technologies for solid-state nanopores reported to date are summarized, with the advantages and limitations of each technology discussed in detail. Advanced shrinking strategies to prepare nanopores with desired shapes and sizes down to sub-1 nm are concluded. Finally, applications of solid-state nanopores in DNA sequencing, single molecule detection, ion-selective transport, and nanopatterning are outlined.
Keywords: applications of solid-state nanopores; fabrication and shrinking technologies; solid-state nanopores.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Controllable Shrinking Fabrication of Solid-State Nanopores.Micromachines (Basel). 2022 Jun 10;13(6):923. doi: 10.3390/mi13060923. Micromachines (Basel). 2022. PMID: 35744537 Free PMC article. Review.
-
Precise electrochemical fabrication of sub-20 nm solid-state nanopores for single-molecule biosensing.J Phys Condens Matter. 2010 Nov 17;22(45):454128. doi: 10.1088/0953-8984/22/45/454128. Epub 2010 Oct 29. J Phys Condens Matter. 2010. PMID: 21339614
-
TEM based applications in solid state nanopores: From fabrication to liquid in-situ bio-imaging.Micron. 2022 Nov;162:103347. doi: 10.1016/j.micron.2022.103347. Epub 2022 Sep 1. Micron. 2022. PMID: 36081256 Review.
-
Shrinking solid-state nanopores using electron-beam-induced deposition.Nanotechnology. 2009 Mar 18;20(11):115302. doi: 10.1088/0957-4484/20/11/115302. Epub 2009 Feb 24. Nanotechnology. 2009. PMID: 19420436
-
Single-Entity Detection With TEM-Fabricated Nanopores.Front Chem. 2021 May 7;9:664820. doi: 10.3389/fchem.2021.664820. eCollection 2021. Front Chem. 2021. PMID: 34026729 Free PMC article. Review.
Cited by
-
Recent advances in integrated solid-state nanopore sensors.Lab Chip. 2021 Aug 21;21(16):3030-3052. doi: 10.1039/d1lc00294e. Epub 2021 Jun 17. Lab Chip. 2021. PMID: 34137407 Free PMC article. Review.
-
Next-Generation Sequencing and Triple-Negative Breast Cancer: Insights and Applications.Int J Mol Sci. 2023 Jun 2;24(11):9688. doi: 10.3390/ijms24119688. Int J Mol Sci. 2023. PMID: 37298642 Free PMC article. Review.
-
The Research Progress of Single-Molecule Sequencing and Its Significance in Nucleic Acid Metrology.Biosensors (Basel). 2024 Dec 25;15(1):4. doi: 10.3390/bios15010004. Biosensors (Basel). 2024. PMID: 39852055 Free PMC article. Review.
-
Fabrication of angstrom-scale two-dimensional channels for mass transport.Nat Protoc. 2024 Jan;19(1):240-280. doi: 10.1038/s41596-023-00911-x. Epub 2023 Nov 27. Nat Protoc. 2024. PMID: 38012396 Review.
-
Solid-State Nanopores for Biomolecular Analysis and Detection.Adv Biochem Eng Biotechnol. 2024;187:283-316. doi: 10.1007/10_2023_240. Adv Biochem Eng Biotechnol. 2024. PMID: 38273209 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

