MSC.sTRAIL Has Better Efficacy than MSC.FL-TRAIL and in Combination with AKTi Blocks Pro-Metastatic Cytokine Production in Prostate Cancer Cells
- PMID: 31010082
- PMCID: PMC6521093
- DOI: 10.3390/cancers11040568
MSC.sTRAIL Has Better Efficacy than MSC.FL-TRAIL and in Combination with AKTi Blocks Pro-Metastatic Cytokine Production in Prostate Cancer Cells
Abstract
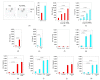
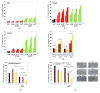
Cell therapy is a promising new treatment option for cancer. In particular, mesenchymal stem cells (MSCs) have shown potential in delivering therapeutic genes in various tumour models and are now on the verge of being tested in the clinic. A number of therapeutic genes have been examined in this context, including the death ligand TRAIL. For cell therapy, it can be used in its natural form as a full-length and membrane-bound protein (FL-TRAIL) or as an engineered version commonly referred to as soluble TRAIL (sTRAIL). As to which is more therapeutically efficacious, contradicting results have been reported. We discovered that MSCs producing sTRAIL have significantly higher apoptosis-inducing activity than cells expressing FL-TRAIL and found that FL-TRAIL, in contrast to sTRAIL, is not secreted. We also demonstrated that TRAIL does induce the expression of pro-metastatic cytokines in prostate cancer cells, but that this effect could be overcome through combination with an AKT inhibitor. Thus, a combination consisting of small-molecule drugs specifically targeting tumour cells in combination with MSC.sTRAIL, not only provides a way of sensitising cancer cells to TRAIL, but also reduces the issue of side-effect-causing cytokine production. This therapeutic strategy therefore represents a novel targeted treatment option for advanced prostate cancer and other difficult to treat tumours.
Keywords: AKT; AKTi; CXCL5; ENA-78; IL-6; cell therapy; mesenchymal stem cells; prostate cancer; sTRAIL.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Delivery of sTRAIL variants by MSCs in combination with cytotoxic drug treatment leads to p53-independent enhanced antitumor effects.Cell Death Dis. 2013 Feb 21;4(2):e503. doi: 10.1038/cddis.2013.19. Cell Death Dis. 2013. PMID: 23429289 Free PMC article.
-
Impact of soluble tumor necrosis factor-related apoptosis-inducing ligand released by engineered adipose mesenchymal stromal cells on white blood cells.Cytotherapy. 2023 Jun;25(6):605-614. doi: 10.1016/j.jcyt.2023.02.008. Epub 2023 Apr 1. Cytotherapy. 2023. PMID: 37012089
-
Targeting of XIAP combined with systemic mesenchymal stem cell-mediated delivery of sTRAIL ligand inhibits metastatic growth of pancreatic carcinoma cells.Stem Cells. 2010 Nov;28(11):2109-20. doi: 10.1002/stem.533. Stem Cells. 2010. PMID: 20882532
-
Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour.Int J Mol Sci. 2018 Jul 27;19(8):2188. doi: 10.3390/ijms19082188. Int J Mol Sci. 2018. PMID: 30060445 Free PMC article. Review.
-
Review: on TRAIL for malignant glioma therapy?Neuropathol Appl Neurobiol. 2010 Apr;36(3):168-82. doi: 10.1111/j.1365-2990.2010.01069.x. Epub 2010 Jan 20. Neuropathol Appl Neurobiol. 2010. PMID: 20102513 Review.
Cited by
-
Harnessing TRAIL-Induced Apoptosis Pathway for Cancer Immunotherapy and Associated Challenges.Front Immunol. 2021 Aug 20;12:699746. doi: 10.3389/fimmu.2021.699746. eCollection 2021. Front Immunol. 2021. Retraction in: Front Immunol. 2023 Sep 04;14:1285126. doi: 10.3389/fimmu.2023.1285126. PMID: 34489946 Free PMC article. Retracted. Review.
-
Thieno[2,3-b]pyridine compounds potently inhibit prostate cancer growth and motility.Endocr Oncol. 2025 Jul 8;5(1):e240082. doi: 10.1530/EO-24-0082. eCollection 2025 Jan. Endocr Oncol. 2025. PMID: 40641632 Free PMC article.
-
Chemo-Sensitization of CD133+ Cancer Stem Cell Enhances the Effect of Mesenchymal Stem Cell Expressing TRAIL in Non-Small Cell Lung Cancer Cell Lines.Biology (Basel). 2021 Oct 26;10(11):1103. doi: 10.3390/biology10111103. Biology (Basel). 2021. PMID: 34827096 Free PMC article.
-
Mesenchymal Stem/Stromal Cells as a Vehicle for Cytokine Delivery: An Emerging Approach for Tumor Immunotherapy.Front Med (Lausanne). 2021 Aug 27;8:721174. doi: 10.3389/fmed.2021.721174. eCollection 2021. Front Med (Lausanne). 2021. Retraction in: Front Med (Lausanne). 2023 Sep 04;10:1285195. doi: 10.3389/fmed.2023.1285195. PMID: 34513882 Free PMC article. Retracted. Review.
-
Identification of the role of autophagy-related TNFSF10/ hsa-let-7a-5p axis in vitiligo development and potential herbs exploring based on a bioinformatics analysis.Heliyon. 2023 Dec 3;9(12):e23220. doi: 10.1016/j.heliyon.2023.e23220. eCollection 2023 Dec. Heliyon. 2023. PMID: 38149194 Free PMC article.
References
-
- Wiley S.R., Schooley K., Smolak P.J., Din W.S., Huang C.P., Nicholl J.K., Sutherland G.R., Smith T.D., Rauch C., Smith C.A., et al. Identification and characterization of a new member of the tnf family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

