Fluoride Exposure Induces Inhibition of Sodium-and Potassium-Activated Adenosine Triphosphatase (Na+, K+-ATPase) Enzyme Activity: Molecular Mechanisms and Implications for Public Health
- PMID: 31010095
- PMCID: PMC6518254
- DOI: 10.3390/ijerph16081427
Fluoride Exposure Induces Inhibition of Sodium-and Potassium-Activated Adenosine Triphosphatase (Na+, K+-ATPase) Enzyme Activity: Molecular Mechanisms and Implications for Public Health
Abstract
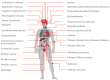
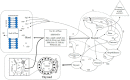
In this study, several lines of evidence are provided to show that Na + , K + -ATPase activity exerts vital roles in normal brain development and function and that loss of enzyme activity is implicated in neurodevelopmental, neuropsychiatric and neurodegenerative disorders, as well as increased risk of cancer, metabolic, pulmonary and cardiovascular disease. Evidence is presented to show that fluoride (F) inhibits Na + , K + -ATPase activity by altering biological pathways through modifying the expression of genes and the activity of glycolytic enzymes, metalloenzymes, hormones, proteins, neuropeptides and cytokines, as well as biological interface interactions that rely on the bioavailability of chemical elements magnesium and manganese to modulate ATP and Na + , K + -ATPase enzyme activity. Taken together, the findings of this study provide unprecedented insights into the molecular mechanisms and biological pathways by which F inhibits Na + , K + -ATPase activity and contributes to the etiology and pathophysiology of diseases associated with impairment of this essential enzyme. Moreover, the findings of this study further suggest that there are windows of susceptibility over the life course where chronic F exposure in pregnancy and early infancy may impair Na + , K + -ATPase activity with both short- and long-term implications for disease and inequalities in health. These findings would warrant considerable attention and potential intervention, not to mention additional research on the potential effects of F intake in contributing to chronic disease.
Keywords: Na+, K+-ATPase; Na+, K+-ATPase and pathological states; cancer; cognitive impairment; fluoride; lung diseases; metabolic diseases; molecular mechanisms of inhibition; neurological diseases.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
-
- Mobasheri A., Avila J., Cózar-Castellano I., Brownleader M.D., Trevan M., Francis M.J., Lamb J.F., Martín-Vasallo P. Na+, K+-ATPase isozyme diversity: Comparative biochemistry and physiological implications of novel functional interactions. Biosci. Rep. 2000;20:51–91. doi: 10.1023/A:1005580332144. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

