miR-194 Accelerates Apoptosis of Aβ1⁻42-Transduced Hippocampal Neurons by Inhibiting Nrn1 and Decreasing PI3K/Akt Signaling Pathway Activity
- PMID: 31010100
- PMCID: PMC6523401
- DOI: 10.3390/genes10040313
miR-194 Accelerates Apoptosis of Aβ1⁻42-Transduced Hippocampal Neurons by Inhibiting Nrn1 and Decreasing PI3K/Akt Signaling Pathway Activity
Abstract
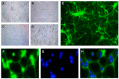
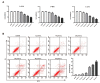
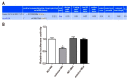
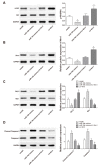
This article explores the mechanism of miR-194 on the proliferation and apoptosis of Aβ1-42-transduced hippocampal neurons. Aβ1-42-transduced hippocampal neuron model was established by inducing hippocampal neurons with Aβ1-42. MTT assay and flow cytometry were used to detect the viability and apoptosis of hippocampal neurons, respectively. qRT-PCR was used to detect changes in miR-194 and Nrn1 expression after Aβ1-42 induction. Aβ1-42-transduced hippocampal neurons were transfected with miR-194 mimics and/or Nrn1 overexpression vectors. Their viability and neurite length were detected by MTT assay and immunofluorescence, respectively. Western blot was used to detect protein expression. Aβ1-42 inhibited Aβ1-42-transduced hippocampal neuron activity and promoted their apoptosis in a dose-dependent manner. miR-194 was upregulated and Nrn1 was downregulated in Aβ1-42-transduced hippocampal neurons (p < 0.05). Compared with the model group, Aβ1-42-transduced hippocampal neurons of the miR-194 mimic group had much lower activity, average longest neurite length, Nrn1, p-AkT, and Bcl-2 protein expression and had much higher Bax, Caspase-3, and Cleaved Caspase-3 protein expression. Compared with the model group, Aβ1-42-transduced hippocampal neurons of the LV-Nrn1 group had much higher activity, average longest neurite length, Nrn1, p-AkT, and Bcl-2 protein expression and had much lower Bax, Caspase-3, and Cleaved Caspase-3 protein expression. Nrn1 is a target gene of miR-194. miR-194 inhibited apoptosis of Aβ1-42-transduced hippocampal neurons by inhibiting Nrn1 and decreasing PI3K/AkT signaling pathway activity.
Keywords: Alzheimer’s disease; Nrn1; PI3K/AkT signaling pathway; apoptosis; hippocampal neurons; miR-194; proliferation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Xu J., Begley P., Church S.J., Patassini S., Hollywood K.A., Jüllig M., Curtis M.A., Waldvogel H.J., Faull R.L.M., Unwin R.D. Graded perturbations of metabolism in multiple regions of human brain in Alzheimer’s disease: Snapshot of a pervasive metabolic disorder. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016;1862:1084–1092. doi: 10.1016/j.bbadis.2016.03.001. - DOI - PMC - PubMed
-
- Förstl H., Sattel H., Bahro M. Alzheimer’s disease: Clinical features. Int. Rev. Psychiatry. 2009;5:327–349. doi: 10.3109/09540269309037797. - DOI
-
- Kim H.J., Cha J., Lee J.M., Shin J.S., Jung N.Y., Kim Y.J., Choe Y.S., Lee K.H., Kim S.T., Kim J.S. Distinctive Resting State Network Disruptions Among Alzheimer’s Disease, Subcortical Vascular Dementia, and Mixed Dementia Patients. Alzheimers Dement. J. Alzheimers Assoc. 2016;11:P412. doi: 10.1016/j.jalz.2015.06.375. - DOI - PubMed
-
- Koyama A., Hashimoto M., Tanaka H., Fujise N., Matsushita M., Miyagawa Y., Hatada Y., Fukuhara R., Hasegawa N., Todani S. Malnutrition in Alzheimer’s Disease, Dementia with Lewy Bodies, and Frontotemporal Lobar Degeneration: Comparison Using Serum Albumin, Total Protein, and Hemoglobin Level. PLoS ONE. 2016;11:e0157053. doi: 10.1371/journal.pone.0157053. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

