Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment
- PMID: 31010180
- PMCID: PMC6523119
- DOI: 10.3390/nano9040638
Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment
Abstract
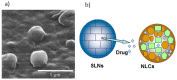
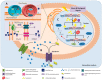
Many therapeutically active molecules are non-soluble in aqueous systems, chemically and biologically fragile or present severe side effects. Lipid-based nanoparticle (LBNP) systems represent one of the most promising colloidal carriers for bioactive organic molecules. Their current application in oncology has revolutionized cancer treatment by improving the antitumor activity of several chemotherapeutic agents. LBNPs advantages include high temporal and thermal stability, high loading capacity, ease of preparation, low production costs, and large-scale industrial production since they can be prepared from natural sources. Moreover, the association of chemotherapeutic agents with lipid nanoparticles reduces active therapeutic dose and toxicity, decreases drug resistance and increases drug levels in tumor tissue by decreasing them in healthy tissue. LBNPs have been extensively assayed in in vitro cancer therapy but also in vivo, with promising results in some clinical trials. This review summarizes the types of LBNPs that have been developed in recent years and the main results when applied in cancer treatment, including essential assays in patients.
Keywords: cancer; clinical trials; drug release; drug resistance; lipid-based nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rama A.R., Jimenez-Lopez J., Cabeza L., Jimenez-Luna C., Leiva M.C., Perazzoli G., Hernandez R., Zafra I., Ortiz R., Melguizo C., et al. Last Advances in Nanocarriers-Based Drug Delivery Systems for Colorectal Cancer. Curr. Drug Deliv. 2016;13:830–838. doi: 10.2174/1567201813666151203232852. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

