Synthesis of an Undecasaccharide Featuring an Oligomannosidic Heptasaccharide and a Bacterial Kdo-lipid A Backbone for Eliciting Neutralizing Antibodies to Mammalian Oligomannose on the HIV-1 Envelope Spike
- PMID: 31010286
- PMCID: PMC6524000
- DOI: 10.1021/jacs.9b02872
Synthesis of an Undecasaccharide Featuring an Oligomannosidic Heptasaccharide and a Bacterial Kdo-lipid A Backbone for Eliciting Neutralizing Antibodies to Mammalian Oligomannose on the HIV-1 Envelope Spike
Abstract
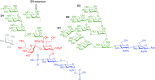
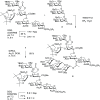
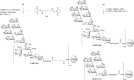
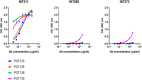
Lipooligosaccharides (LOS) from the bacterium Rhizobium radiobacter Rv3 are structurally related to antigenic mammalian oligomannoses on the HIV-1 envelope glycoprotein spike that are targets for broadly neutralizing antibodies. Here, we prepared a hybrid structure of viral and bacterial epitopes as part of a vaccine design strategy to elicit oligomannose-specific HIV-neutralizing antibodies using glycoconjugates based on the Rv3 LOS structure. Starting from a Kdo2GlcNAc2 tetrasaccharide precursor, a central orthogonally protected mannose trichloroacetimidate donor was coupled to OH-5 of the innermost Kdo residue. To assemble larger glycans, the N-acetylamino groups of the glucosamine units were converted to imides to prevent formation of unwanted imidate byproducts. Blockwise coupling of the pentasaccharide acceptor with an α-(1→2)-linked mannotriosyl trichloroacetimidate donor introduced the D1-arm fragment. Glycosylation of O-6 of the central branching mannose with an α-(1→2)-α-(1→6)-linked mannotriosyl trichloroacetimidate donor unit then furnished the undecasaccharide harboring a D3-arm extension. Global deprotection yielded the 3-aminopropyl ligand, which was activated as an isothiocyanate or adipic acid succinimidoyl ester and conjugated to CRM197. However, representative oligomannose-specific HIV-neutralizing antibodies bound the undecasaccharide conjugates poorly. Possible reasons for this outcome are discussed herein along with paths for improvement.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

