An Inward-Rectifier Potassium Channel Coordinates the Properties of Biologically Derived Membranes
- PMID: 31010661
- PMCID: PMC6510706
- DOI: 10.1016/j.bpj.2019.03.023
An Inward-Rectifier Potassium Channel Coordinates the Properties of Biologically Derived Membranes
Abstract
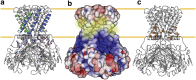
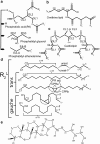
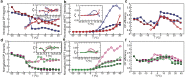
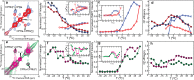
KirBac1.1 is a prokaryotic inward-rectifier K+ channel from Burkholderia pseudomallei. It shares the common inward-rectifier K+ channel fold with eukaryotic channels, including conserved lipid-binding pockets. Here, we show that KirBac1.1 changes the phase properties and dynamics of the surrounding bilayer. KirBac1.1 was reconstituted into vesicles composed of 13C-enriched biological lipids. Two-dimensional liquid-state and solid-state NMR experiments were used to assign lipid 1H and 13C chemical shifts as a function of lipid identity and conformational degrees of freedom. A solid-state NMR temperature series reveals that KirBac1.1 lowers the primary thermotropic phase transition of Escherichia coli lipid membranes while introducing both fluidity and internal lipid order into the fluid phases. In B. thailandensis liposomes, the bacteriohopanetetrol hopanoid, and potentially ornithine lipids, introduce a similar primary lipid-phase transition and liquid-ordered properties. Adding KirBac1.1 to B. thailandensis lipids increases B. thailandensis lipid fluidity while preserving internal lipid order. This synergistic effect of KirBac1.1 in bacteriohopanetetrol-rich membranes has implications for bilayer dynamic structure. If membrane proteins can anneal lipid translational degrees of freedom while preserving internal order, it could offer an explanation to the nature of liquid-ordered protein-lipid organization in vivo.
Copyright © 2019. Published by Elsevier Inc.
Figures





Similar articles
-
Conformational changes upon gating of KirBac1.1 into an open-activated state revealed by solid-state NMR and functional assays.Proc Natl Acad Sci U S A. 2020 Feb 11;117(6):2938-2947. doi: 10.1073/pnas.1915010117. Epub 2020 Jan 24. Proc Natl Acad Sci U S A. 2020. PMID: 31980523 Free PMC article.
-
Crystal structure of the potassium channel KirBac1.1 in the closed state.Science. 2003 Jun 20;300(5627):1922-6. doi: 10.1126/science.1085028. Epub 2003 May 8. Science. 2003. PMID: 12738871
-
Conformational changes at cytoplasmic intersubunit interactions control Kir channel gating.J Biol Chem. 2017 Jun 16;292(24):10087-10096. doi: 10.1074/jbc.M117.785154. Epub 2017 Apr 26. J Biol Chem. 2017. PMID: 28446610 Free PMC article.
-
Lipids driving protein structure? Evolutionary adaptations in Kir channels.Channels (Austin). 2010 May-Jun;4(3):139-41. doi: 10.4161/chan.4.3.12129. Epub 2010 May 1. Channels (Austin). 2010. PMID: 21150302 Free PMC article. Review.
-
Regulation of cardiac inwardly rectifying potassium channels by membrane lipid metabolism.Prog Biophys Mol Biol. 2003 Jan;81(1):67-79. doi: 10.1016/s0079-6107(02)00048-2. Prog Biophys Mol Biol. 2003. PMID: 12475570 Review.
Cited by
-
NMR studies of lipid regulation of the K+ channel KcsA.Biochim Biophys Acta Biomembr. 2021 Mar 1;1863(3):183491. doi: 10.1016/j.bbamem.2020.183491. Epub 2020 Oct 13. Biochim Biophys Acta Biomembr. 2021. PMID: 33065136 Free PMC article.
-
2H,13C-Cholesterol for Dynamics and Structural Studies of Biological Membranes.ACS Omega. 2022 May 10;7(20):17151-17160. doi: 10.1021/acsomega.2c00796. eCollection 2022 May 24. ACS Omega. 2022. PMID: 35647452 Free PMC article.
-
Codon Harmonization of a Kir3.1-KirBac1.3 Chimera for Structural Study Optimization.Biomolecules. 2020 Mar 10;10(3):430. doi: 10.3390/biom10030430. Biomolecules. 2020. PMID: 32164257 Free PMC article.
-
Spectroscopic signatures of bilayer ordering in native biological membranes.Biochim Biophys Acta Biomembr. 2022 Jun 1;1864(6):183891. doi: 10.1016/j.bbamem.2022.183891. Epub 2022 Feb 22. Biochim Biophys Acta Biomembr. 2022. PMID: 35217001 Free PMC article.
-
The Lipid Activation Mechanism of a Transmembrane Potassium Channel.J Am Chem Soc. 2020 Aug 19;142(33):14102-14116. doi: 10.1021/jacs.0c01991. Epub 2020 Aug 7. J Am Chem Soc. 2020. PMID: 32702990 Free PMC article.
References
-
- Brown D.A., London E. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem. Biophys. Res. Commun. 1997;240:1–7. - PubMed
-
- Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. - PubMed
-
- Simons K., Vaz W.L. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004;33:269–295. - PubMed
-
- Lee A.G. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta. 2003;1612:1–40. - PubMed
-
- Brown M.F. Soft matter in lipid-protein interactions. Annu. Rev. Biophys. 2017;46:379–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

