Engineering the TGFβ Receptor to Enhance the Therapeutic Potential of Natural Killer Cells as an Immunotherapy for Neuroblastoma
- PMID: 31010834
- PMCID: PMC6635028
- DOI: 10.1158/1078-0432.CCR-18-3183
Engineering the TGFβ Receptor to Enhance the Therapeutic Potential of Natural Killer Cells as an Immunotherapy for Neuroblastoma
Abstract
Purpose: The ability of natural killer (NK) cells to lyse allogeneic targets, without the need for explicit matching or priming, makes them an attractive platform for cell-based immunotherapy. Umbilical cord blood is a practical source for generating banks of such third-party NK cells for "off-the-shelf" cell therapy applications. NK cells are highly cytolytic, and their potent antitumor effects can be rapidly triggered by a lack of HLA expression on interacting target cells, as is the case for a majority of solid tumors, including neuroblastoma. Neuroblastoma is a leading cause of pediatric cancer-related deaths and an ideal candidate for NK-cell therapy. However, the antitumor efficacy of NK cells is limited by immunosuppressive cytokines in the tumor microenvironment, such as TGFβ, which impair NK cell function and survival.
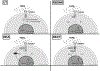
Experimental design: To overcome this, we genetically modified NK cells to express variant TGFβ receptors, which couple a mutant TGFβ dominant-negative receptor to NK-specific activating domains. We hypothesized that with these engineered receptors, inhibitory TGFβ signals are effectively converted to activating signals.
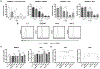
Results: Modified NK cells exhibited higher cytotoxic activity against neuroblastoma in a TGFβ-rich environment in vitro and superior progression-free survival in vivo, as compared with their unmodified controls.
Conclusions: Our results support the development of "off-the-shelf" gene-modified NK cells, that overcome TGFβ-mediated immune evasion, in patients with neuroblastoma and other TGFβ-secreting malignancies.
©2019 American Association for Cancer Research.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures




References
-
- Tey SK, Bollard CM, Heslop HE, Adoptive T-cell transfer in cancer immunotherapy. Immunol Cell Biol 84, 281–289 (2006). - PubMed
-
- Herberman RB, Nunn ME, Holden HT, Lavrin DH, Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer 16, 230–239 (1975). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

