Role of Caulobacter Cell Surface Structures in Colonization of the Air-Liquid Interface
- PMID: 31010900
- PMCID: PMC6707911
- DOI: 10.1128/JB.00064-19
Role of Caulobacter Cell Surface Structures in Colonization of the Air-Liquid Interface
Abstract
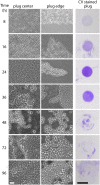
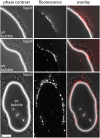
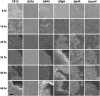
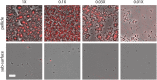
In aquatic environments, Caulobacter spp. can be found at the boundary between liquid and air known as the neuston. I report an approach to study temporal features of Caulobacter crescentus colonization and pellicle biofilm development at the air-liquid interface and have defined the role of cell surface structures in this process. At this interface, C. crescentus initially forms a monolayer of cells bearing a surface adhesin known as the holdfast. When excised from the liquid surface, this monolayer strongly adheres to glass. The monolayer subsequently develops into a three-dimensional structure that is highly enriched in clusters of stalked cells known as rosettes. As this pellicle film matures, it becomes more cohesive and less adherent to a glass surface. A mutant strain lacking a flagellum does not efficiently reach the surface, and strains lacking type IV pili exhibit defects in organization of the three-dimensional pellicle. Strains unable to synthesize the holdfast fail to accumulate at the boundary between air and liquid and do not form a pellicle. Phase-contrast images support a model whereby the holdfast functions to trap C. crescentus cells at the air-liquid boundary. Unlike the holdfast, neither the flagellum nor type IV pili are required for C. crescentus to partition to the air-liquid interface. While it is well established that the holdfast enables adherence to solid surfaces, this study provides evidence that the holdfast has physicochemical properties that allow partitioning of nonmotile mother cells to the air-liquid interface and facilitate colonization of this microenvironment.IMPORTANCE In aquatic environments, the boundary at the air interface is often highly enriched with nutrients and oxygen. Colonization of this niche likely confers a significant fitness advantage in many cases. This study provides evidence that the cell surface adhesin known as a holdfast enables Caulobacter crescentus to partition to and colonize the air-liquid interface. Additional surface structures, including the flagellum and type IV pili, are important determinants of colonization and biofilm formation at this boundary. Considering that holdfast-like adhesins are broadly conserved in Caulobacter spp. and other members of the diverse class Alphaproteobacteria, these surface structures may function broadly to facilitate colonization of air-liquid boundaries in a range of ecological contexts, including freshwater, marine, and soil ecosystems.
Keywords: Alphaproteobacteria; Caulobacter; biofilm; flagellum; holdfast; neuston; pellicle; type 4 pilus; unipolar polysaccharide.
Copyright © 2019 American Society for Microbiology.
Figures






References
-
- Davies JT, Rideal EK. 1963. Interfacial phenomena, 2nd ed Academic Press, London, United Kingdom.
-
- Van Oss CJ. 2006. Interfacial forces in aqueous media, 2nd ed, p 438 Taylor & Francis, Boca Raton, FL.
-
- Marshall KC. 1996. Adhesion as a strategy for access to nutrients, p 59–87. In Fletcher M (ed), Bacterial adhesion: molecular and ecological diversity. Wiley-Liss, Inc, New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

