Photoelectric conversion on Earth's surface via widespread Fe- and Mn-mineral coatings
- PMID: 31010932
- PMCID: PMC6525476
- DOI: 10.1073/pnas.1902473116
Photoelectric conversion on Earth's surface via widespread Fe- and Mn-mineral coatings
Abstract
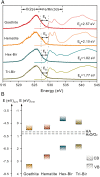
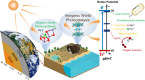
Sunlight drives photosynthesis and associated biological processes, and also influences inorganic processes that shape Earth's climate and geochemistry. Bacterial solar-to-chemical energy conversion on this planet evolved to use an intricate intracellular process of phototrophy. However, a natural nonbiological counterpart to phototrophy has yet to be recognized. In this work, we reveal the inherent "phototrophic-like" behavior of vast expanses of natural rock/soil surfaces from deserts, red soils, and karst environments, all of which can drive photon-to-electron conversions. Using scanning electron microscopy, transmission electron microscopy, micro-Raman spectroscopy, and X-ray absorption spectroscopy, Fe and Mn (oxyhydr)oxide-rich coatings were found in rock varnishes, as were Fe (oxyhydr)oxides on red soil surfaces and minute amounts of Mn oxides on karst rock surfaces. By directly fabricating a photoelectric detection device on the thin section of a rock varnish sample, we have recorded an in situ photocurrent micromapping of the coatings, which behave as highly sensitive and stable photoelectric systems. Additional measurements of red soil and powder separated from the outermost surface of karst rocks yielded photocurrents that are also sensitive to irradiation. The prominent solar-responsive capability of the phototrophic-like rocks/soils is ascribed to the semiconducting Fe- and Mn (oxyhydr)oxide-mineral coatings. The native semiconducting Fe/Mn-rich coatings may play a role similar, in part, to photosynthetic systems and thus provide a distinctive driving force for redox (bio)geochemistry on Earth's surfaces.
Keywords: birnessite; mineral coatings; phototrophic; redox (bio)geochemistry; solar energy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Foukal P, Fröhlich C, Spruit H, Wigley TML. Variations in solar luminosity and their effect on the Earth’s climate. Nature. 2006;443:161–166. - PubMed
-
- Dau H, Zaharieva I. Principles, efficiency, and blueprint character of solar-energy conversion in photosynthetic water oxidation. Acc Chem Res. 2009;42:1861–1870. - PubMed
-
- Katan J. Solar heating (solarization) of soil for control of soilborne pests. Annu Rev Phytopathol. 1981;19:211–236.
-
- Des Marais DJ. When did photosynthesis emerge on Earth? Science. 2000;289:1703–1705. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

