Biosynthesis, regulation, and engineering of microbially produced branched biofuels
- PMID: 31011367
- PMCID: PMC6461809
- DOI: 10.1186/s13068-019-1424-9
Biosynthesis, regulation, and engineering of microbially produced branched biofuels
Abstract
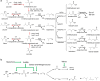
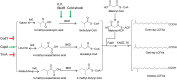
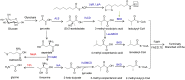
The steadily increasing demand on transportation fuels calls for renewable fuel replacements. This has attracted a growing amount of research to develop advanced biofuels that have similar physical, chemical, and combustion properties with petroleum-derived fossil fuels. Early generations of biofuels, such as ethanol, butanol, and straight-chain fatty acid-derived esters or hydrocarbons suffer from various undesirable properties and can only be blended in limited amounts. Recent research has shifted to the production of branched-chain biofuels that, compared to straight-chain fuels, have higher octane values, better cold flow, and lower cloud points, making them more suitable for existing engines, particularly for diesel and jet engines. This review focuses on several types of branched-chain biofuels and their immediate precursors, including branched short-chain (C4-C8) and long-chain (C15-C19)-alcohols, alkanes, and esters. We discuss their biosynthesis, regulation, and recent efforts in their overproduction by engineered microbes.
Keywords: Advanced biofuels; Branched alcohols; Branched fatty acids; Branched fuels; Cyclopropane fatty acid.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

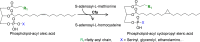
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

