A collaborative, academic approach to optimizing the national clinical research infrastructure: The first year of the Trial Innovation Network
- PMID: 31011433
- PMCID: PMC6474372
- DOI: 10.1017/cts.2018.319
A collaborative, academic approach to optimizing the national clinical research infrastructure: The first year of the Trial Innovation Network
Abstract
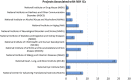
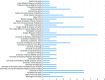
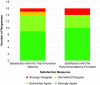
Inefficiencies in the national clinical research infrastructure have been apparent for decades. The National Center for Advancing Translational Science (NCATS) -sponsored CTSA program is able to address such inefficiencies. The Trial Innovation Network (TIN) is a collaborative initiative with the CTSA Program and other NIH Institutes and Centers (ICs) that addresses critical roadblocks to accelerate the translation of novel interventions to clinical practice. The TIN's mission is to execute high quality trials in a quick, cost-efficient manner. The TIN awardees are composed of: three Trial Innovation Centers (TICs), the Recruitment Innovation Center (RIC), and the individual CTSA institutions that have identified TIN Liaison units. The TIN has launched a national scale single (central) IRB system, master contracting agreements, quality-by-design approaches, novel recruitment support methods, and applies evidence-based strategies to recruitment and patient engagement. The TIN has received 113 submissions from 39 different CTSA institutions and 8 non-CTSA Institutions, with projects associated with 12 different NIH ICs across a wide range of clinical/disease areas. Already more than 150 unique health systems/organizations are involved as sites in TIN related multisite studies. The TIN will begin to capture data and metrics that quantify increased efficiency and quality improvement during operations.
Keywords: CTSA program; Trial Innovation Network; clinical trials; innovation; multisite clinical research.
Conflict of interest statement
Disclosure statement: No conflicts of interest exist.
Figures

References
-
- Peters S. Tufts CSDD Assessment of Cost to Develop and Win Marketing Approval for a New Drug Now Published. Tufts Center for the Study of Drug Development News [Internet], 2016 [cited May 6, 2016]. (http://csdd.tufts.edu/news/complete_story/tufts_csdd_rd_cost_study_now_p...)
-
- Sertkaya A, et al. Examination of Clinical Trial Costs and Barriers for Drug Development. Lexington, MA: Eastern Research Group, Inc, 2014.
-
- Zerhouni EA. Translational and clinical science–time for a new vision. New England Journal of Medicine 2005; 353: 1621–1623. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
