Production and stability study of a hospital parenteral nutrition solution for neonates
- PMID: 31011464
- PMCID: PMC6460230
- DOI: 10.1016/j.jpha.2018.01.002
Production and stability study of a hospital parenteral nutrition solution for neonates
Abstract
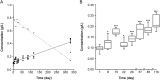
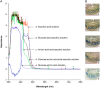
Standard parenteral nutrition solutions are mixtures comprising interacting components that may degrade themselves over time. The objective of this study was to investigate the physicochemical and microbiological stability of a hospital preparation for parenteral nutrition in neonatology. The analyses were performed throughout the storage of the preparations at 2-8 °C (up to 4 months). The extent of stability was based on the determination of amino acids dosage, visual and physicochemical properties (glucose and electrolytes concentrations, pH and osmolality measurements, particle counting) and microbiological analysis (sterility test). A thermal degradation of ascorbic acid was conducted to evaluate the antioxidant properties of the parenteral mixture. Physicochemical and microbiological controls were found to comply with the specifications. Amino acids showed a good stability throughout the 4months storage except for cysteine, which was progressively degraded to cystine, conferring a yellow coloration to parenteral solutions. Parenteral nutrition standards solutions remain stable for 4 months at 2-8 °C, ensuring safe administration in preterm infants.
Keywords: Amino acids; High-performance liquid chromatographic; Neonatology; Parenteral nutrition; Stability-indicating method study.
Figures
References
-
- Baudouin A., Diouf E., Tall M.L. Advantages and special features of hospital preparations of parenteral nutrition in neonatology. Ann. Pharm. Fr. 2015;73:150–159. - PubMed
-
- Callan J., Salvestrini C. Parenteral Nutrition in paediatrics. Paediatr. Child Health. 2013;23:356–361.
-
- Koletzko B., Goulet O., Hunt J. Guidelines on paediatric parenteral nutrition of the European society of paediatric gastroenterology, hepatology and nutrition (ESPGHAN) and the European society for clinical nutrition and metabolism (ESPEN), supported by the European society of paediatric research (ESPR) J. Pediatr. Gastroenterol. Nutr. 2005;41(Suppl. 2):S1–S87. - PubMed
-
- Singe P., Berge M.M., van den Bergh G. ESPEN guidelines on parenteral nutrition: intensive care. Clin. Nutr. 2009;28:387–400. - PubMed
-
- Lapillonne A., Berleur M.P., Brasseur Y. Safety of parenteral nutrition in newborns: results from a nationwide prospective cohort study. Clin. Nutr. 2018;37:624–629. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

