Enantioselective Box Behenken Optimized HPLC-DAD Method for the Simultaneous Estimation of Alogliptin Enantiomorphs in Pharmaceutical Formulations and their Pharmacokinetic Study in Rat Plasma
- PMID: 31011569
- PMCID: PMC6468233
- DOI: 10.15171/apb.2019.018
Enantioselective Box Behenken Optimized HPLC-DAD Method for the Simultaneous Estimation of Alogliptin Enantiomorphs in Pharmaceutical Formulations and their Pharmacokinetic Study in Rat Plasma
Abstract
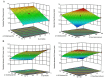
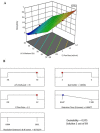
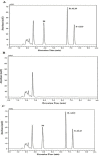
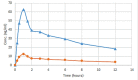
Purpose: A stereoselective high performance liquid chromatographic analytical method with photodiode array detector was developed and validated as per the International Conference on Harmonization (ICH) guidelines for the determination of alogliptin (ALO) enantiomers in formulations and rat plasma. Methods: Enantiomeric separation was performed on a Phenomenex Lux Cellulose-2 chiral column. Box-Behnken design was used to identify the optimum conditions of the three independent variables for the desired output responses. Results: The HPLC peaks of ALO enantiomers and the internal standard pioglitazone were achieved before 8 min with a resolution of 0.77 min between R and S enantiomer and resolution of more than 2.0 between each enantiomer and pioglitazone (internal) with more than 95% recovery. The linearity range and the limit of quantification of both the enantiomers in rat plasma were 10-70 ng mL-1 and 1.2 ng mL-1 respectively. Conclusion: The developed method after validation was successfully applied for estimation of ALO enantiomers in formulations. Single oral dose of 25 mg of the ALO racemate tablets were administered to a group of 6 healthy rats for a comparative pharmacokinetic study of both the enantiomers.
Keywords: Alogliptin enantiomers; Box–Behnken design; HPLC-DAD; Pharmacokinetics; SPE.
Figures
Similar articles
-
Enantioselective HPLC-DAD method for the determination of etodolac enantiomers in tablets, human plasma and application to comparative pharmacokinetic study of both enantiomers after a single oral dose to twelve healthy volunteers.Talanta. 2014 Dec;130:506-17. doi: 10.1016/j.talanta.2014.07.011. Epub 2014 Jul 11. Talanta. 2014. PMID: 25159440
-
Chiral liquid chromatography resolution and stereoselective pharmacokinetic study of pioglitazone enantiomers in rats.J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Mar 1;951-952:143-8. doi: 10.1016/j.jchromb.2014.01.013. Epub 2014 Jan 16. J Chromatogr B Analyt Technol Biomed Life Sci. 2014. PMID: 24561309
-
Development and Validation of a Chiral Liquid Chromatographic Assay for Enantiomeric Separation and Quantification of Verapamil in Rat Plasma: Stereoselective Pharmacokinetic Application.Molecules. 2021 Apr 6;26(7):2091. doi: 10.3390/molecules26072091. Molecules. 2021. PMID: 33917412 Free PMC article.
-
Development and validation of LC/MS method for the determination of meclizine enantiomers in pharmaceutical formulations.Drug Dev Ind Pharm. 2021 Mar;47(3):361-366. doi: 10.1080/03639045.2020.1862174. Epub 2021 Feb 19. Drug Dev Ind Pharm. 2021. PMID: 33291999
-
Simultaneous enantiomer determination of 20 (R)- and 20 (S)-ginsenoside-Rg2 in rat plasma after intravenous administration using HPLC method.J Chromatogr B Analyt Technol Biomed Life Sci. 2007 May 1;850(1-2):1-6. doi: 10.1016/j.jchromb.2006.11.008. Epub 2006 Nov 20. J Chromatogr B Analyt Technol Biomed Life Sci. 2007. PMID: 17113840 Review.
Cited by
-
A new strategy for the determination of the antidiabetics alogliptin, saxagliptin and vildagliptin using all-solid state potentiometric sensors.BMC Chem. 2023 Jul 16;17(1):79. doi: 10.1186/s13065-023-00988-1. BMC Chem. 2023. PMID: 37455315 Free PMC article.
-
An overview of chiral separations of pharmaceutically active substances by HPLC (2018-2020).Monatsh Chem. 2021;152(9):1033-1043. doi: 10.1007/s00706-021-02832-5. Epub 2021 Aug 24. Monatsh Chem. 2021. PMID: 34456367 Free PMC article. Review.
References
-
- Bhutani R, Pathak DP, Kapoor G, Husain A, Kant R, Iqbal MA. Synthesis, molecular modelling studies and ADME prediction of benzothiazole clubbed oxadiazole-Mannich bases, and evaluation of their anti-diabetic activity through in vivo model. Bioorg Chem. 2018;77:6–15. doi: 10.1016/j.bioorg.2017.12.037. - DOI - PubMed
-
- Kapoor G, Pathak DP, Bhutani R, Kant R. Thiazolidinone as a pharmacologically active molecule. J Chem Pharm Res. 2016;8(4):151–68.
-
- Christopher R, Covington P, Davenport M, Fleck P, Mekki QA, Wann ER. et al. Pharmacokinetics, pharmacodynamics, and tolerability of single increasing doses of the dipeptidyl peptidase-4 inhibitor alogliptin in healthy male subjects. Clin Ther. 2008;30(3):513–27. doi: 10.1016/j.clinthera.2008.03.005. - DOI - PubMed
-
- Covington P, Christopher R, Davenport M, Fleck P, Mekki QA, Wann ER. et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin Ther. 2008;30(3):499–512. doi: 10.1016/j.clinthera.2008.03.004. - DOI - PubMed
LinkOut - more resources
Full Text Sources