BAY 1213790, a fully human IgG1 antibody targeting coagulation factor XIa: First evaluation of safety, pharmacodynamics, and pharmacokinetics
- PMID: 31011708
- PMCID: PMC6462747
- DOI: 10.1002/rth2.12186
BAY 1213790, a fully human IgG1 antibody targeting coagulation factor XIa: First evaluation of safety, pharmacodynamics, and pharmacokinetics
Abstract
Background: Coagulation factor XI (FXI) contributes to the development of thrombosis but appears to play only a minor role in hemostasis and is therefore an attractive anticoagulant drug target.
Objectives: To evaluate the safety, pharmacodynamic, and pharmacokinetic properties of BAY 1213790, a fully human immunoglobulin (Ig) G1 antibody targeting activated coagulation FXI (FXIa), in healthy men.
Methods: In this phase 1, single-blind, parallel-group, placebo-controlled, dose-escalation study, 83 healthy Caucasian men were randomized 4:1 to receive a single 60-minute intravenous infusion of BAY 1213790 (0.015-10 mg/kg) or placebo. Adverse events, pharmacodynamic parameters (including activated partial thromboplastin time [aPTT]) and pharmacokinetic parameters were determined. Volunteers were followed up for 150 days.
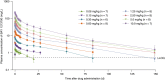
Results: BAY 1213790 demonstrated favorable safety and tolerability; there were no observed cases of bleeding or clinically relevant antidrug antibody formation. One volunteer (1.2%) experienced an infusion reaction. Following intravenous administration of BAY 1213790, dose-dependent increases in aPTT (maximal mean increase relative to baseline: 1.85 [conventional method] and 2.17 [kaolin-triggered method]) and rotational thromboelastometry whole blood clotting time were observed, as well as dose-dependent reductions in FXI activity. Bleeding times did not increase following administration of BAY 1213790 and were similar for all dose cohorts, including placebo. Measurable and dose-dependent increases in systemic exposure were detected for all doses of BAY 1213790 of 0.06 mg/kg or higher.
Conclusions: Based on these safety, pharmacodynamic, and pharmacokinetic results, further evaluation of BAY 1213790 in patients with, or at risk of, thrombosis is warranted.
Keywords: anticoagulant; factor XI; hemostasis; phase 1; thrombosis.
Figures



Similar articles
-
First evaluation of the safety, pharmacokinetics, and pharmacodynamics of BAY 2433334, a small molecule targeting coagulation factor XIa.J Thromb Haemost. 2021 Oct;19(10):2407-2416. doi: 10.1111/jth.15439. Epub 2021 Jul 19. J Thromb Haemost. 2021. PMID: 34192419 Free PMC article. Clinical Trial.
-
First randomized evaluation of safety, pharmacodynamics, and pharmacokinetics of BAY 1831865, an antibody targeting coagulation factor XI and factor XIa, in healthy men.J Thromb Haemost. 2022 Jul;20(7):1684-1695. doi: 10.1111/jth.15744. Epub 2022 May 20. J Thromb Haemost. 2022. PMID: 35490404 Free PMC article. Clinical Trial.
-
Pharmacokinetics, pharmacodynamics and safety of BAY 2433334, a novel activated factor XI inhibitor, in healthy volunteers: A randomized phase 1 multiple-dose study.Br J Clin Pharmacol. 2022 Jul;88(7):3447-3462. doi: 10.1111/bcp.15230. Epub 2022 Mar 24. Br J Clin Pharmacol. 2022. PMID: 35014061 Free PMC article. Clinical Trial.
-
Development of new anticoagulant in 2023: Prime time for anti-factor XI and XIa inhibitors.J Med Vasc. 2023 Apr;48(2):69-80. doi: 10.1016/j.jdmv.2023.04.002. Epub 2023 May 5. J Med Vasc. 2023. PMID: 37422330 Review.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
Cited by
-
Improved efficacy/safety profile of factor XIa inhibitor BMS-724296 versus factor Xa inhibitor apixaban and thrombin inhibitor dabigatran in cynomolgus monkeys.Res Pract Thromb Haemost. 2021 May 28;5(4):e12524. doi: 10.1002/rth2.12524. eCollection 2021 May. Res Pract Thromb Haemost. 2021. PMID: 34095733 Free PMC article.
-
Factor XI, a potential target for anticoagulation therapy for venous thromboembolism.Front Cardiovasc Med. 2022 Oct 31;9:975767. doi: 10.3389/fcvm.2022.975767. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36386334 Free PMC article. Review.
-
Pharmacological profile of asundexian, a novel, orally bioavailable inhibitor of factor XIa.J Thromb Haemost. 2022 Jun;20(6):1400-1411. doi: 10.1111/jth.15700. Epub 2022 Mar 25. J Thromb Haemost. 2022. PMID: 35289054 Free PMC article.
-
Factor XI inhibitors for the prevention and treatment of venous and arterial thromboembolism.Nat Rev Cardiol. 2025 Mar 31. doi: 10.1038/s41569-025-01144-z. Online ahead of print. Nat Rev Cardiol. 2025. PMID: 40164778 Review.
-
Novel horizons in anticoagulation: the emerging role of factor XI inhibitors across different settings.Haematologica. 2024 Oct 1;109(10):3110-3124. doi: 10.3324/haematol.2023.283682. Haematologica. 2024. PMID: 38779744 Free PMC article. Review.
References
-
- Zhang H, Lowenberg EC, Crosby JR, MacLeod AR, Zhao C, Gao D, et al. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood. 2010;116:4684–92. - PubMed
-
- Preis M, Hirsch J, Kotler A, Zoabi A, Stein N, Rennert G, et al. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood. 2017;129:1210–5. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

