Polyethylene glycol 3350 plus electrolytes for chronic constipation: a 2-week, randomized, double-blind, placebo-controlled study with a 52-week open-label extension
- PMID: 31011797
- PMCID: PMC6698298
- DOI: 10.1007/s00535-019-01581-x
Polyethylene glycol 3350 plus electrolytes for chronic constipation: a 2-week, randomized, double-blind, placebo-controlled study with a 52-week open-label extension
Abstract
Background: Although polyethylene glycol 3350 plus electrolytes (PEG3350 + E) is the most widely used osmotic laxative in Europe, prospective data on its long-term (over 6 months) safety and efficacy are not available to date.
Methods: Japanese patients with chronic constipation were randomized to receive PEG3350 + E or placebo for 2 weeks orally. Following this, the patients received PEG3350 + E in the 52-week extension study. The starting dose was 13.7 g/day dissolved in 125 mL of water, and dose titration was allowed (upper limit 41.1 g/day) according to the patient's bowel condition. The primary efficacy endpoint was the change from baseline in frequency of spontaneous bowel movements (SBMs) at week 2 in the double-blind study. Secondary endpoints and adverse events were assessed. Safety and efficacy were also assessed in the extension study.
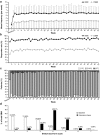
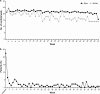
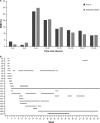
Results: Among 204 patients who provided informed consent, 156 were randomized and included in the full analysis. The frequency of SBMs was significantly higher with PEG3350 + E [least squares mean (LSM) 4.3, 95% confidence interval (CI) 3.6-4.9] compared with placebo (LSM 1.6, 95% CI 1.2-2.1; P < 0.0001). A total of 153 patients entered the extension study; PEG3350 + E led to a sustained improvement in bowel function. The common adverse drug reactions during the entire study period were mild gastrointestinal disorders (abdominal pain 4.5%, diarrhea 3.8%, nausea 3.2%, abdominal distension 2.6%).
Conclusions: Treatment with PEG3350 + E resolved constipation in the short term, was well tolerated, and led to sustained improvement in bowel function in the long-term treatment of Japanese patients with chronic constipation.
Clinical trial registration number: Japic CTI-163167.
Keywords: Chronic constipation; Polyethylene glycol 3350 plus electrolytes; Prospective long-term clinical trial; Spontaneous bowel movement.
Conflict of interest statement
AN has served as an advisor to EA Pharma Co., Ltd. KS and AO are employees of EA Pharma Co., Ltd. AN and YK have received lecture fees from EA Pharma Co., Ltd. YK has received fees for writing promotional material for EA Pharma Co., Ltd. AN and YK have received a research grant from EA Pharma Co., Ltd.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

