Sustained-release of sclerostin single-chain antibody fragments using poly(lactic-co-glycolic acid) microspheres for osteoporotic fracture repair
- PMID: 31012249
- PMCID: PMC6618085
- DOI: 10.1002/jbm.a.36704
Sustained-release of sclerostin single-chain antibody fragments using poly(lactic-co-glycolic acid) microspheres for osteoporotic fracture repair
Abstract
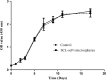
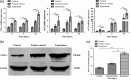
Osteoporotic fracture is one of the most common bone diseases in middle and old age, as the most serious consequence of osteoporosis. Sclerostin single-chain antibody fragments (SCL-scFv) have been proven to promote bone formation by binding to scleroprotein, a natural antagonist of the Wnt pathway, but it is difficult to rule alone due to the weak permeability and immunogenicity. Herein, we prepared poly(lactic-co-glycolic acid) microspheres as a sustained-release vehicle to prolong the activity of SCL-scFv. The morphology of microspheres were uniform and nearly sphere, loading efficiency and encapsulation efficiency of SCL-scFv microspheres were 6.28 ± 1.04% and 48.37 ± 8.11%, respectively. Approximately 90% of the SCL-scFvs were released from the microspheres over 28 days with initial burst releasing (38%) in the first 4 days. Sustained-release of active SCL-scFv from microspheres promoted bone marrow mesenchymal stem cells osteogenic differentiation in vitro and enhanced fracture healing in ovariectomized rats by improving bone mass and bone formation in the fracture region. All these findings demonstrate that the microspheres are able to simultaneously achieve localized long-term SCL-scFv controlled release and effectively promote bone formation, which provides a promising approach for osteoporotic fracture.
Keywords: fracture healing; microspheres; osteoporosis; protein release; sclerostin-scFv.
© 2019 The Authors. Journal of Biomedical Materials Research Part A published by Wiley Periodicals, Inc.
Figures




Similar articles
-
Dexamethasone Long-Term Controlled Release from Injectable Dual-Network Hydrogels with Porous Microspheres Immunomodulation Promotes Bone Regeneration.ACS Appl Mater Interfaces. 2024 Aug 7;16(31):40581-40601. doi: 10.1021/acsami.4c06661. Epub 2024 Jul 29. ACS Appl Mater Interfaces. 2024. PMID: 39074361 Free PMC article.
-
Sustained release of melatonin from poly (lactic-co-glycolic acid) (PLGA) microspheres to induce osteogenesis of human mesenchymal stem cells in vitro.J Pineal Res. 2013 Jan;54(1):24-32. doi: 10.1111/j.1600-079X.2012.01016.x. Epub 2012 Jun 19. J Pineal Res. 2013. PMID: 22712496
-
Expression of sclerostin scFv and the effect of sclerostin scFv on healing of osteoporotic femur fracture in rats.Cell Biochem Biophys. 2014 Jun;69(2):229-35. doi: 10.1007/s12013-013-9787-1. Cell Biochem Biophys. 2014. PMID: 24254971
-
Fracture healing in osteoporotic bone.Injury. 2016 Jun;47 Suppl 2:S21-6. doi: 10.1016/S0020-1383(16)47004-X. Injury. 2016. PMID: 27338222 Review.
-
Inhibitors of sclerostin: emerging concepts.Curr Opin Rheumatol. 2014 Jul;26(4):447-52. doi: 10.1097/BOR.0000000000000073. Curr Opin Rheumatol. 2014. PMID: 24807403 Free PMC article. Review.
Cited by
-
Association between Geriatric Nutritional Risk Index and all-cause mortality in individuals with osteoporotic fractures: a retrospective cohort study.Aging Clin Exp Res. 2025 Mar 11;37(1):77. doi: 10.1007/s40520-025-02978-w. Aging Clin Exp Res. 2025. PMID: 40069532 Free PMC article.
References
-
- Asmus, L. R. , Grimshaw, J. P. , Richle, P. , Eicher, B. , Urech, D. M. , Gurny, R. , & Möller, M. (2015). Injectable formulations for an intravitreal sustained‐release application of a novel single‐chain VEGF antibody fragment. European Journal of Pharmaceutics and Biopharmaceutics, 95(Pt. B), 250–260. - PubMed
-
- Cheung, W. H. , Miclau, T. , Chow, S. K. , Yang, F. F. , & Alt, V. (2016). Fracture healing in osteoporotic bone. Injury, 47(Suppl. 2), S21–S26. - PubMed
-
- Cummings, S. R. , & Melton, L. J. (2002). Epidemiology and outcomes of osteoporotic fractures. Lancet, 359(9319), 1761–1767. - PubMed
-
- Esbrit, P. , & Alcaraz, M. J. (2013). Current perspectives on parathyroid hormone (PTH) and PTH‐related protein (PTHrP) as bone anabolic therapies. Biochemical Pharmacology, 85(10), 1417–1423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2017FC-TSYS-2006/Clinical Research Support Fund of PLA General Hospital/International
- AWS17J004/Subsidiary of PLA Major Project/International
- 81702153/National Natural Science Foundation of China/International
- 81702121/National Natural Science Foundation of China/International
- 2018-4-5014/Scientific Research Project of Capital Health Development/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

