Induction of Inflammation and Fibrosis by Semaphorin 4A in Systemic Sclerosis
- PMID: 31012544
- PMCID: PMC6790618
- DOI: 10.1002/art.40915
Induction of Inflammation and Fibrosis by Semaphorin 4A in Systemic Sclerosis
Abstract
Objective: To analyze the potential role of semaphorin 4A (Sema4A) in inflammatory and fibrotic processes involved in the pathology of systemic sclerosis (SSc).
Methods: Sema4A levels in the plasma of healthy controls (n = 11) and SSc patients (n = 20) were determined by enzyme-linked immunosorbent assay (ELISA). The expression of Sema4A and its receptors in monocytes and CD4+ T cells from healthy controls and SSc patients (n = 6-7 per group) was determined by ELISA and flow cytometry. Th17 cytokine production by CD4+ T cells (n = 5-7) was analyzed by ELISA and flow cytometry. The production of inflammatory mediators and extracellular matrix (ECM) components by dermal fibroblast cells (n = 6) was analyzed by quantitative polymerase chain reaction, ELISA, Western blotting, confocal microscopy, and ECM deposition assay.
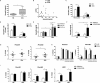
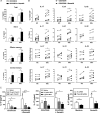
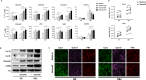
Results: Plasma levels of Sema4A, and Sema4A expression by circulating monocytes and CD4+ T cells, were significantly higher in SSc patients than in healthy controls (P < 0.05). Inflammatory mediators significantly up-regulated the secretion of Sema4A by monocytes and CD4+ T cells from SSc patients (P < 0.05 versus unstimulated SSc cells). Functional assays showed that Sema4A significantly enhanced the expression of Th17 cytokines induced by CD3/CD28 in total CD4+ T cells as well in different CD4+ T cell subsets (P < 0.05 versus unstimulated SSc cells). Finally, Sema4A induced a profibrotic phenotype in dermal fibroblasts from both healthy controls and SSc patients, which was abrogated by blocking or silencing the expression of Sema4A receptors.
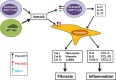
Conclusion: Our findings indicate that Sema4A plays direct and dual roles in promoting inflammation and fibrosis, 2 main features of SSc, suggesting that Sema4A might be a novel therapeutic target in SSc.
© 2019 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures


References
-
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009;360:1989–2003. - PubMed
-
- Denton CP, Khanna D. Systemic sclerosis. Lancet 2017;390:1685–99. - PubMed
-
- Van Bon L, Cossu M, Radstake TR. An update on an immune system that goes awry in systemic sclerosis. Curr Opin Rheumatol 2011;23:505–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

