Intra-islet glucagon signaling is critical for maintaining glucose homeostasis
- PMID: 31012868
- PMCID: PMC6542600
- DOI: 10.1172/jci.insight.127994
Intra-islet glucagon signaling is critical for maintaining glucose homeostasis
Abstract
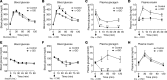
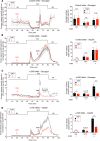
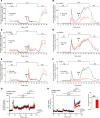
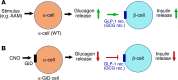
Glucagon, a hormone released from pancreatic alpha-cells, plays a key role in maintaining proper glucose homeostasis and has been implicated in the pathophysiology of diabetes. In vitro studies suggest that intra-islet glucagon can modulate the function of pancreatic beta-cells. However, because of the lack of suitable experimental tools, the in vivo physiological role of this intra-islet cross-talk has remained elusive. To address this issue, we generated a novel mouse model that selectively expressed an inhibitory designer G protein-coupled receptor (Gi DREADD) in α-cells only. Drug-induced activation of this inhibitory designer receptor almost completely shut off glucagon secretion in vivo, resulting in significantly impaired insulin secretion, hyperglycemia, and glucose intolerance. Additional studies with mouse and human islets indicated that intra-islet glucagon stimulates insulin release primarily by activating β-cell GLP-1 receptors. These new findings strongly suggest that intra-islet glucagon signaling is essential for maintaining proper glucose homeostasis in vivo. Our work may pave the way toward the development of novel classes of antidiabetic drugs that act by modulating intra-islet cross-talk between α- and β-cells.
Keywords: G-protein coupled receptors; Glucose metabolism; Islet cells; Metabolism.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

