Randomized Double-Blind Phase II Study of Regorafenib in Patients With Metastatic Osteosarcoma
- PMID: 31013172
- PMCID: PMC7799443
- DOI: 10.1200/JCO.18.02374
Randomized Double-Blind Phase II Study of Regorafenib in Patients With Metastatic Osteosarcoma
Abstract
Purpose: SARC024 is a phase II clinical trial of the multikinase inhibitor regorafenib in specific sarcoma subtypes, including advanced osteosarcoma. We hypothesized that regorafenib would improve progression-free survival (PFS) in patients with sarcoma and report the results of the osteosarcoma cohort.
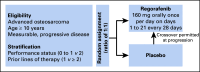
Patients and methods: This trial enrolled patients with progressive metastatic osteosarcoma with measurable disease by RECIST who had received at least one prior line of therapy. Patients were randomly assigned at a ratio of one to one to regorafenib or placebo. Crossover was allowed at time of disease progression. PFS was the primary end point of the study, which was powered to detect a difference of at least 3 months in median PFS.
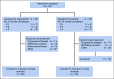
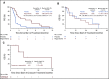
Results: Forty-two patients from 12 centers were enrolled between September 2014 and May 2018. Median age was 37 years (range, 18 to 76 years). Patients had received an average of 2.3 prior therapy regimens. Ten patients receiving placebo crossed over to active drug at time of progression. Study enrollment was stopped early, after a data safety monitoring committee review. Median PFS was significantly improved with regorafenib versus placebo: 3.6 months (95% CI, 2.0 to 7.6 months) versus 1.7 months (95% CI, 1.2 to 1.8 months), respectively (hazard ratio, 0.42; 95% CI, 0.21 to 0.85; P = .017). In the context of the crossover design, there was no statistically significant difference in overall survival. Fourteen (64%) of 22 patients initially randomly assigned to regorafenib experienced grade 3 to 4 events attributed to treatment, including one grade 4 colonic perforation.
Conclusion: The study met its primary end point, demonstrating activity of regorafenib in patients with progressive metastatic osteosarcoma. No new safety signals were observed. Regorafenib should be considered a treatment option for patients with relapsed metastatic osteosarcoma.
Trial registration: ClinicalTrials.gov NCT02048371.
Conflict of interest statement
Randomized Double-Blind Phase II Study of Regorafenib in Patients With Metastatic Osteosarcoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Lara E. Davis
Christopher W. Ryan
Kristen N. Ganjoo
Elizabeth T. Loggers
Sant Chawla
Mark Agulnik
Damon Reed
Vicky Keedy
Daniel Rushing
Denise K. Reinke
Richard F. Riedel
Steven Attia
Leo Mascarenhas
Robert G. Maki
No other potential conflicts of interest were reported.
Figures
References
-
- Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity N Engl J Med 3141600–16061986 - PubMed
-
- Bielack SS, Smeland S, Whelan JS, et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: First results of the EURAMOS-1 good response randomized controlled trial J Clin Oncol 332279–22872015 - PMC - PubMed
-
- Whelan JS, Davis LE.Osteosarcoma, chondrosarcoma, and chordoma J Clin Oncol 36188–1932018 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

