Randomized Phase II Trial to Compare the Efficacy of Haloperidol and Olanzapine in the Control of Chemotherapy-Induced Nausea and Vomiting in Nepal
- PMID: 31013182
- PMCID: PMC6528728
- DOI: 10.1200/JGO.18.00245
Randomized Phase II Trial to Compare the Efficacy of Haloperidol and Olanzapine in the Control of Chemotherapy-Induced Nausea and Vomiting in Nepal
Abstract
Purpose: The purpose of the study was to compare efficacy and toxicity of olanzapine (OLN; a higher-cost drug) and haloperidol (HAL; a lower-cost drug) in the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients who receive highly emetogenic chemotherapy (HEC).
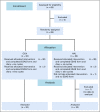
Patients and methods: In a randomized, phase II trial, patients were randomly assigned to receive either OLN 10 mg orally on days 1 to 4 or HAL 1 mg orally on day 1 and 0.5 mg twice daily on days 2 to 4. Both groups received ondansetron 16 mg and dexamethasone 12 mg intravenously on day 1. Patients recorded their nausea using the Edmonton Symptom Assessment Scale (ESAS) and recorded daily episodes of vomiting from day 1 to day 5. The primary end point was complete nausea prevention (CNP; ie, ESAS of 0). Secondary end point was complete emesis prevention (CEP).
Results: Sixty-five patients were randomly assigned, and 64 received their allocated treatment (n = 32 in each arm). There was no difference in CNP during the overall period (days 1 to 5) between OLN and HAL (68.7% v 71.8%; P = .78). In the acute period (day 1) and the delayed period (days 2 to 5), CNP was similar between OLN and HAL (acute: 84.3% v 81.2%; delayed: 68.7% v 75%). No difference was identified in the rate of CEP during the overall period (81.2% with OLN v 78.1% with HAL; P = .75), during the acute period (93.7% with OLN v 90.6% with HAL), or during the delayed period (84.3% with OLN v 84.3% with HAL). No difference in toxicities was noted between treatment arms.
Conclusion: In this study, HAL had comparable efficacy to OLN in the management of CINV, which suggests that it is the higher-value option in patients who receive HEC in resource-scarce countries.
Conflict of interest statement
Randomized Phase II Trial to Compare the Efficacy of Haloperidol and Olanzapine in the Control of Chemotherapy-Induced Nausea and Vomiting in Nepal
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Prakash Neupane
Lori Anne Wood
No other potential conflicts of interest were reported.
Figures
Similar articles
-
Randomized, double-blind, placebo-controlled study of aprepitant versus two dosages of olanzapine with ondansetron plus dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving high-emetogenic chemotherapy.Support Care Cancer. 2020 Nov;28(11):5335-5342. doi: 10.1007/s00520-020-05380-6. Epub 2020 Mar 4. Support Care Cancer. 2020. PMID: 32128615 Clinical Trial.
-
Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting: A Comparative Study From Sudan.J Glob Oncol. 2018 Sep;4:1-9. doi: 10.1200/JGO.17.00216. J Glob Oncol. 2018. PMID: 30241275 Free PMC article.
-
Efficacy and safety of rolapitant for prevention of chemotherapy-induced nausea and vomiting over multiple cycles of moderately or highly emetogenic chemotherapy.Eur J Cancer. 2016 Apr;57:23-30. doi: 10.1016/j.ejca.2015.12.023. Epub 2016 Feb 4. Eur J Cancer. 2016. PMID: 26851398 Clinical Trial.
-
Randomized, Placebo-Controlled, Phase III Trial of Fosaprepitant, Ondansetron, Dexamethasone (FOND) Versus FOND Plus Olanzapine (FOND-O) for the Prevention of Chemotherapy-Induced Nausea and Vomiting in Patients with Hematologic Malignancies Receiving Highly Emetogenic Chemotherapy and Hematopoietic Cell Transplantation Regimens: The FOND-O Trial.Biol Blood Marrow Transplant. 2018 Oct;24(10):2065-2071. doi: 10.1016/j.bbmt.2018.06.005. Epub 2018 Jun 13. Biol Blood Marrow Transplant. 2018. PMID: 29906570 Clinical Trial.
-
Efficacy of olanzapine for quality of life improvement among patients with malignant tumor: A systematic review.Cancer Rep (Hoboken). 2019 Aug;2(4):e1167. doi: 10.1002/cnr2.1167. Epub 2019 Feb 26. Cancer Rep (Hoboken). 2019. PMID: 32721128 Free PMC article.
Cited by
-
Antiemetic drugs: what to prescribe and when.Aust Prescr. 2020 Apr;43(2):49-56. doi: 10.18773/austprescr.2020.011. Epub 2020 Apr 1. Aust Prescr. 2020. PMID: 32346211 Free PMC article. Review.
-
Efficacy of Olanzapine for High and Moderate Emetogenic Chemotherapy in Children.Children (Basel). 2020 Sep 16;7(9):140. doi: 10.3390/children7090140. Children (Basel). 2020. PMID: 32948015 Free PMC article.
-
Olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting: a systematic review, meta-analysis, cumulative meta-analysis and fragility assessment of the literature.Support Care Cancer. 2021 Jul;29(7):3439-3459. doi: 10.1007/s00520-020-05935-7. Epub 2021 Jan 13. Support Care Cancer. 2021. PMID: 33442782 Free PMC article.
References
-
- Cohen L, de Moor CA, Eisenberg P, et al. Chemotherapy-induced nausea and vomiting: Incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007;15:497–503. - PubMed
-
- Laszlo J: Emesis as limiting toxicity in cancer chemotherapy, in Laszlo J (ed): Antiemetics and Cancer Chemotherapy. Baltimore, MD, Williams & Wilkins, 1983, pp 1-5.
-
- Fernández-Ortega P, Caloto MT, Chirveches E, et al. Chemotherapy-induced nausea and vomiting in clinical practice: Impact on patients’ quality of life. Support Care Cancer. 2012;20:3141–3148. - PubMed
-
- Kris MG, Gralla RJ, Clark RA, et al. Incidence, course, and severity of delayed nausea and vomiting following the administration of high-dose cisplatin. J Clin Oncol. 1985;3:1379–1384. - PubMed
-
- Roila F, Boschetti E, Tonato M, et al. Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone: A randomized single-blind study. Am J Clin Oncol. 1991;14:238–242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical