Critical role of Interleukin 21 and T follicular helper cells in hypertension and vascular dysfunction
- PMID: 31013256
- PMCID: PMC6629096
- DOI: 10.1172/jci.insight.129278
Critical role of Interleukin 21 and T follicular helper cells in hypertension and vascular dysfunction
Abstract
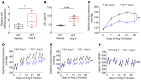
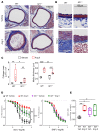
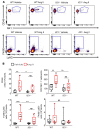
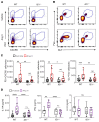
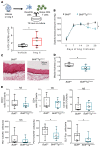
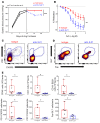
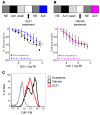
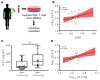
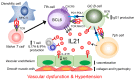
T and B cells have been implicated in hypertension, but the mechanisms by which they produce a coordinated response is unknown. T follicular helper (Tfh) cells that produce interleukin 21 (IL21) promote germinal center (GC) B cell responses leading to immunoglobulin (Ig) production. Here we investigate the role of IL21 and Tfh cells in hypertension. In response to angiotensin (Ang) II-induced hypertension, T cell IL21 production is increased, and Il21-/- mice develop blunted hypertension, attenuated vascular end-organ damage, and decreased interleukin 17A (IL17A) and interferon gamma production. Tfh-like cells and GC B cells accumulate in the aorta and plasma IgG1 is increased in hypertensive WT but not Il21-/-mice. Furthermore, Tfh cell deficient mice develop blunted hypertension and vascular hypertrophy in response to Ang II infusion. Importantly, IL21 neutralization reduces blood pressure (BP) and reverses endothelial dysfunction and vascular inflammation. Moreover, recombinant IL21 impairs endothelium-dependent relaxation ex vivo and decreases nitric oxide production from cultured endothelial cells. Finally, we show in humans that peripheral blood T cell production of IL21 correlates with systolic BP and IL17A production. These data suggest that IL21 may be a novel therapeutic target for the treatment of hypertension and its micro- and macrovascular complications.
Keywords: Adaptive immunity; Cardiology; Cardiovascular disease; Immunology; T cells.
Conflict of interest statement
Figures

References
-
- Whelton PK, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high BP in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066. - DOI - PubMed
-
- GBD 2015 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8. - DOI - PMC - PubMed
-
- Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;null(133):1–8. - PubMed
-
- Okuda T, Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med. 1967;25(2):257–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 HL144048/HL/NHLBI NIH HHS/United States
- S10 OD023475/OD/NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- I01 BX000915/BX/BLRD VA/United States
- DP2 HL137166/HL/NHLBI NIH HHS/United States
- T32 HL069765/HL/NHLBI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- P01 HL129941/HL/NHLBI NIH HHS/United States
- F32 HL143927/HL/NHLBI NIH HHS/United States
- K08 HL121671/HL/NHLBI NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- T32 HL007411/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

