Targeting self and neo-epitopes with a modular self-adjuvanting cancer vaccine
- PMID: 31013258
- PMCID: PMC6629093
- DOI: 10.1172/jci.insight.127305
Targeting self and neo-epitopes with a modular self-adjuvanting cancer vaccine
Abstract
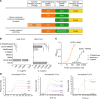
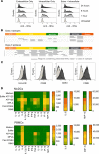
Induction of a potent CD4 and CD8 T-cell response against tumor-specific and tumor-associated antigen is critical for eliminating tumor cells. Recent vaccination strategies have been hampered by an inefficacious and low amplitude immune response. Here we describe a self-adjuvanted chimeric protein vaccine platform to address these challenges, characterized by a multidomain construction incorporating (i) a cell penetrating peptide (CPP) allowing internalization of several multiantigenic Major Histocompatibility Complex (MHC)-restricted peptides within (ii) the multiantigenic domain (Mad) and (iii) a TLR2/4 agonist domain (TLRag). Functionality of the resulting chimeric protein is based on the combined effect of the above-mentioned three different domains for simultaneous activation of antigen presenting cells and antigen cross-presentation, leading to an efficacious multiantigenic and multiallelic cellular immune response. Helper and cytotoxic T-cell responses were observed against model-, neo- and self-antigens, and were highly potent in several murine tumor models. The safety and the immunogenicity of a human vaccine candidate designed for colorectal cancer treatment was demonstrated in a non-human primate model. This newly engineered therapeutic vaccine approach is promising for the treatment of poorly infiltrated tumors that do not respond to currently marketed immunotherapies.
Keywords: Cellular immune response; Colorectal cancer; Immunotherapy; Oncology; Vaccines.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

