Fibroblast activation and inflammation in frozen shoulder
- PMID: 31013287
- PMCID: PMC6478286
- DOI: 10.1371/journal.pone.0215301
Fibroblast activation and inflammation in frozen shoulder
Abstract
Introduction: Frozen shoulder is a common, fibro-proliferative disease characterised by the insidious onset of pain and progressively restricted range of shoulder movement. Despite the prevalence of this disease, there is limited understanding of the molecular mechanisms underpinning the pathogenesis of this debilitating disease. Previous studies have identified increased myofibroblast differentiation and proliferation, immune cell influx and dysregulated cytokine production. We hypothesised that subpopulations within the fibroblast compartment may take on an activated phenotype, thus initiating the inflammatory processes observed in frozen shoulder. Therefore, we sought to evaluate the presence and possible pathogenic role of known stromal activation proteins in Frozen shoulder.
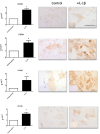
Methods: Shoulder capsule samples were collected from 10 patients with idiopathic frozen shoulder and 10 patients undergoing shoulder stabilisation surgery. Fibroblast activation marker expression (CD248, CD146, VCAM and PDPN, FAP) was quantified using immunohistochemistry. Control and diseased fibroblasts were cultured for in vitro studies from capsule biopsies from instability and frozen shoulder surgeries, respectively. The inflammatory profile and effects of IL-1β upon diseased and control fibroblasts was assessed using ELISA, immunohistochemistry and qPCR.
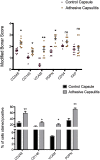
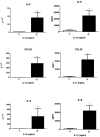
Results: Immunohistochemistry demonstrated increased expression of fibroblast activation markers CD248, CD146, VCAM and PDPN in the frozen shoulder group compared with control (p < 0.05). Fibroblasts cultured from diseased capsule produced elevated levels of inflammatory protein (IL-6, IL-8 & CCL-20) in comparison to control fibroblasts. Exposing control fibroblasts to an inflammatory stimuli, (IL-1ß) significantly increased stromal activation marker transcript and protein expression (CD248, PDPN and VCAM).
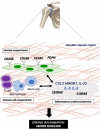
Conclusions: These results show that fibroblasts have an activated phenotype in frozen shoulder and this is associated with inflammatory cytokine dysregulation. Furthermore, it supports the hypothesis that activated fibroblasts may be involved in regulating the inflammatory and fibrotic processes involved in this disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

