Disruption of Structural Disulfides of Coagulation FXIII-B Subunit; Functional Implications for a Rare Bleeding Disorder
- PMID: 31013569
- PMCID: PMC6514982
- DOI: 10.3390/ijms20081956
Disruption of Structural Disulfides of Coagulation FXIII-B Subunit; Functional Implications for a Rare Bleeding Disorder
Abstract
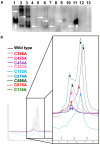
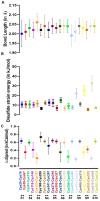
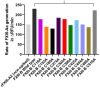
Congenital FXIII deficiency is a rare bleeding disorder in which mutations are detected in F13A1 and F13B genes that express the two subunits of coagulation FXIII, the catalytic FXIII-A, and protective FXIII-B. Mutations in FXIII-B subunit are considerably rarer compared to FXIII-A. Three mutations in the F13B gene have been reported on its structural disulfide bonds. In the present study, we investigate the structural and functional importance of all 20 structural disulfide bonds in FXIII-B subunit. All disulfide bonds were ablated by individually mutating one of its contributory cysteine's, and these variants were transiently expressed in HEK293t cell lines. The expression products were studied for stability, secretion, the effect on oligomeric state, and on FXIII-A activation. The structural flexibility of these disulfide bonds was studied using classical MD simulation performed on a FXIII-B subunit monomer model. All 20 FXIII-B were found to be important for the secretion and stability of the protein since ablation of any of these led to a secretion deficit. However, the degree of effect that the disruption of disulfide bond had on the protein differed between individual disulfide bonds reflecting a functional hierarchy/diversity within these disulfide bonds.
Keywords: FXIII deficiency; FXIII-B; coagulation Factor XIII; disulfide bonds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Identification of Potential Novel Interacting Partners for Coagulation Factor XIII B (FXIII-B) Subunit, a Protein Associated with a Rare Bleeding Disorder.Int J Mol Sci. 2019 May 31;20(11):2682. doi: 10.3390/ijms20112682. Int J Mol Sci. 2019. PMID: 31159152 Free PMC article.
-
Factor XIII: novel structural and functional aspects.J Thromb Haemost. 2011 Jan;9(1):9-20. doi: 10.1111/j.1538-7836.2010.04070.x. J Thromb Haemost. 2011. PMID: 20880254 Review.
-
The Plasma Factor XIII Heterotetrameric Complex Structure: Unexpected Unequal Pairing within a Symmetric Complex.Biomolecules. 2019 Nov 21;9(12):765. doi: 10.3390/biom9120765. Biomolecules. 2019. PMID: 31766577 Free PMC article.
-
In vitro secretion deficits are common among human coagulation factor XIII subunit B missense mutants: correlations with patient phenotypes and molecular models.Hum Mutat. 2013 Nov;34(11):1490-500. doi: 10.1002/humu.22391. Epub 2013 Sep 13. Hum Mutat. 2013. PMID: 23913518
-
Factor XIII: congenital deficiency factor XIII, acquired deficiency, factor XIII A-subunit, and factor XIII B-subunit.Arch Pathol Lab Med. 2014 Feb;138(2):278-81. doi: 10.5858/arpa.2012-0639-RS. Arch Pathol Lab Med. 2014. PMID: 24476525 Review.
Cited by
-
Identification of Potential Novel Interacting Partners for Coagulation Factor XIII B (FXIII-B) Subunit, a Protein Associated with a Rare Bleeding Disorder.Int J Mol Sci. 2019 May 31;20(11):2682. doi: 10.3390/ijms20112682. Int J Mol Sci. 2019. PMID: 31159152 Free PMC article.
-
Intein-mediated recombinant expression of monomeric B22Asp desB30 insulin.BMC Biotechnol. 2020 Jan 9;20(1):3. doi: 10.1186/s12896-020-0598-3. BMC Biotechnol. 2020. PMID: 31918694 Free PMC article.
References
-
- Biswas A., Ivaskevicius V., Thomas A., Oldenburg J. Coagulation factor XIII deficiency. Diagnosis, prevalence and management of inherited and acquired forms. Hamostaseologie. 2014;34:160–166. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

