Identification of ADAM12 as a Novel Basigin Sheddase
- PMID: 31013576
- PMCID: PMC6514901
- DOI: 10.3390/ijms20081957
Identification of ADAM12 as a Novel Basigin Sheddase
Abstract
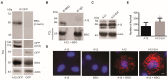
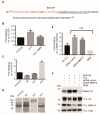
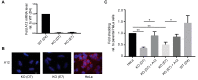
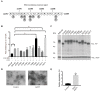
The transmembrane glycoprotein basigin, a member of the immunoglobulin superfamily, stimulates matrix metalloproteinase (MMP)-mediated extracellular matrix (ECM) degradation and thereby drives cancer cell invasion. Basigin is proteolytically shed from the cell surface and high concentrations of soluble basigin in the blood dictates poor prognosis in cancer patients. A positive correlation between basigin and a disintegrin and metalloproteinase (ADAM)-12 in serum from prostate cancer patients has been reported. Yet, the functional relevance of this correlation is unknown. Here, we show that ADAM12 interacts with basigin and cleaves it in the juxtamembrane region. Specifically, overexpression of ADAM12 increases ectodomain shedding of an alkaline phosphatase-tagged basigin reporter protein from the cell surface. Moreover, CRISPR/Cas9-mediated knockout of ADAM12 in human HeLa carcinoma cells results in reduced shedding of the basigin reporter, which can be rescued by ADAM12 re-expression. We detected endogenous basigin fragments, corresponding to the expected size of the ADAM12-generated ectodomain, in conditioned media from ADAM12 expressing cancer cell-lines, as well as serum samples from a healthy pregnant donor and five bladder cancer patients, known to contain high ADAM12 levels. Supporting the cancer relevance of our findings, we identified several cancer-associated mutations in the basigin membrane proximal region. Subsequent in vitro expression showed that some of these mutants are more prone to ADAM12-mediated shedding and that the shed ectodomain can enhance gelatin degradation by cancer cells. In conclusion, we identified ADAM12 as a novel basigin sheddase with a potential implication in cancer.
Keywords: CD147; EMMPRIN; a disintegrin and metalloproteinase; ectodomain shedding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
ADAM12-Generated Basigin Ectodomain Binds β1 Integrin and Enhances the Expression of Cancer-Related Extracellular Matrix Proteins.Int J Mol Sci. 2024 May 28;25(11):5871. doi: 10.3390/ijms25115871. Int J Mol Sci. 2024. PMID: 38892056 Free PMC article.
-
EMMPRIN and ADAM12 in prostate cancer: preliminary results of a prospective study.Tumour Biol. 2014 Nov;35(11):11647-53. doi: 10.1007/s13277-014-2514-8. Epub 2014 Aug 20. Tumour Biol. 2014. PMID: 25139103
-
ADAM12 is expressed in the tumour vasculature and mediates ectodomain shedding of several membrane-anchored endothelial proteins.Biochem J. 2013 May 15;452(1):97-109. doi: 10.1042/BJ20121558. Biochem J. 2013. PMID: 23458101
-
From putative brain tumor marker to high cognitive abilities: Emerging roles of a disintegrin and metalloprotease (ADAM) 12 in the brain.J Chem Neuroanat. 2020 Nov;109:101846. doi: 10.1016/j.jchemneu.2020.101846. Epub 2020 Jul 2. J Chem Neuroanat. 2020. PMID: 32622867 Review.
-
Structural insights on druggable hotspots in CD147: A bull's eye view.Life Sci. 2019 May 1;224:76-87. doi: 10.1016/j.lfs.2019.03.044. Epub 2019 Mar 20. Life Sci. 2019. PMID: 30904494 Review.
Cited by
-
The prominent role of the S100A8/S100A9-CD147 axis in the progression of penile cancer.Front Oncol. 2022 Oct 11;12:891511. doi: 10.3389/fonc.2022.891511. eCollection 2022. Front Oncol. 2022. PMID: 36303837 Free PMC article.
-
HLA-G neo-expression modifies genetic programs governing tumor cell lines.Cancer Immunol Immunother. 2024 Oct 3;73(12):247. doi: 10.1007/s00262-024-03768-5. Cancer Immunol Immunother. 2024. PMID: 39358558 Free PMC article.
-
Novel long non-coding RNA CYB561-5 promotes aerobic glycolysis and tumorigenesis by interacting with basigin in non-small cell lung cancer.J Cell Mol Med. 2022 Mar;26(5):1402-1412. doi: 10.1111/jcmm.17057. Epub 2022 Jan 22. J Cell Mol Med. 2022. PMID: 35064752 Free PMC article.
-
Transmembrane Protease Serine 11B Modulates Lactate Transport Through SLC16A1 in Pancreatic Ductal Adenocarcinoma-A Functional Link to Phenotype Heterogeneity.Int J Mol Sci. 2025 Jun 4;26(11):5398. doi: 10.3390/ijms26115398. Int J Mol Sci. 2025. PMID: 40508207 Free PMC article.
-
Soluble CD147 (BSG) as a Prognostic Marker in Multiple Myeloma.Curr Issues Mol Biol. 2022 Jan 14;44(1):350-359. doi: 10.3390/cimb44010026. Curr Issues Mol Biol. 2022. PMID: 35723405 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

