Molecular Recognition of the Hybrid-Type G-Quadruplexes in Human Telomeres
- PMID: 31013622
- PMCID: PMC6514847
- DOI: 10.3390/molecules24081578
Molecular Recognition of the Hybrid-Type G-Quadruplexes in Human Telomeres
Abstract
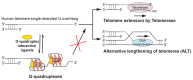
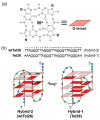
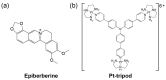
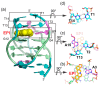
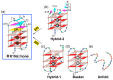
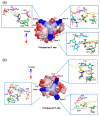
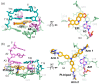
G-quadruplex (G4) DNA secondary structures formed in human telomeres have been shown to inhibit cancer-specific telomerase and alternative lengthening of telomere (ALT) pathways. Thus, human telomeric G-quadruplexes are considered attractive targets for anticancer drugs. Human telomeric G-quadruplexes are structurally polymorphic and predominantly form two hybrid-type G-quadruplexes, namely hybrid-1 and hybrid-2, under physiologically relevant solution conditions. To date, only a handful solution structures are available for drug complexes of human telomeric G-quadruplexes. In this review, we will describe two recent solution structural studies from our labs. We use NMR spectroscopy to elucidate the solution structure of a 1:1 complex between a small molecule epiberberine and the hybrid-2 telomeric G-quadruplex, and the structures of 1:1 and 4:2 complexes between a small molecule Pt-tripod and the hybrid-1 telomeric G-quadruplex. Structural information of small molecule complexes can provide important information for understanding small molecule recognition of human telomeric G-quadruplexes and for structure-based rational drug design targeting human telomeric G-quadruplexes.
Keywords: G-quadruplex; G4; anticancer drug; epi-berberine; human telomeres; hybrid-1; hybrid-2; molecular recognition; platinum-tripod; rational drug design; solution structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
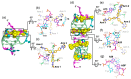
Similar articles
-
Molecular Recognition of the Hybrid-2 Human Telomeric G-Quadruplex by Epiberberine: Insights into Conversion of Telomeric G-Quadruplex Structures.Angew Chem Int Ed Engl. 2018 Aug 20;57(34):10888-10893. doi: 10.1002/anie.201804667. Epub 2018 Jul 18. Angew Chem Int Ed Engl. 2018. PMID: 29888501 Free PMC article.
-
Human Telomeric G-Quadruplex Structures and G-Quadruplex-Interactive Compounds.Methods Mol Biol. 2017;1587:171-196. doi: 10.1007/978-1-4939-6892-3_17. Methods Mol Biol. 2017. PMID: 28324509 Free PMC article.
-
Structure of the Hybrid-2 type intramolecular human telomeric G-quadruplex in K+ solution: insights into structure polymorphism of the human telomeric sequence.Nucleic Acids Res. 2007;35(15):4927-40. doi: 10.1093/nar/gkm522. Epub 2007 Jul 10. Nucleic Acids Res. 2007. PMID: 17626043 Free PMC article.
-
Methods of studying telomere damage induced by quadruplex-ligand complexes.Methods. 2012 May;57(1):93-9. doi: 10.1016/j.ymeth.2012.02.010. Epub 2012 Mar 4. Methods. 2012. PMID: 22410593 Review.
-
G-Quadruplexes at Telomeres: Friend or Foe?Molecules. 2020 Aug 13;25(16):3686. doi: 10.3390/molecules25163686. Molecules. 2020. PMID: 32823549 Free PMC article. Review.
Cited by
-
G-Quadruplexes in Human Telomere: Structures, Properties, and Applications.Molecules. 2023 Dec 27;29(1):174. doi: 10.3390/molecules29010174. Molecules. 2023. PMID: 38202757 Free PMC article. Review.
-
Benzimidazole-based small molecules as anticancer agents targeting telomeric G-quadruplex and inhibiting telomerase enzyme.Future Med Chem. 2024;16(19):2043-2067. doi: 10.1080/17568919.2024.2400982. Epub 2024 Sep 24. Future Med Chem. 2024. PMID: 39316718 Review.
-
BG4 antibody can recognize telomeric G-quadruplexes harboring destabilizing base modifications and lesions.Nucleic Acids Res. 2024 Feb 28;52(4):1763-1778. doi: 10.1093/nar/gkad1209. Nucleic Acids Res. 2024. PMID: 38153143 Free PMC article.
-
The Epigenomic Features and Potential Functions of PEG- and PDS-Favorable DNA G-Quadruplexes in Rice.Int J Mol Sci. 2024 Jan 4;25(1):634. doi: 10.3390/ijms25010634. Int J Mol Sci. 2024. PMID: 38203805 Free PMC article.
-
G-quadruplex Stabilization Fuels the ALT Pathway in ALT-positive Osteosarcoma Cells.Genes (Basel). 2020 Mar 13;11(3):304. doi: 10.3390/genes11030304. Genes (Basel). 2020. PMID: 32183119 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

