Chondroitin Sulfate Safety and Quality
- PMID: 31013685
- PMCID: PMC6515237
- DOI: 10.3390/molecules24081447
Chondroitin Sulfate Safety and Quality
Abstract
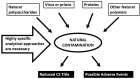
The industrial production of chondroitin sulfate (CS) uses animal tissue sources as raw material derived from different terrestrial or marine species of animals. CS possesses a heterogeneous structure and physical-chemical profile in different species and tissues, responsible for the various and more specialized functions of these macromolecules. Moreover, mixes of different animal tissues and sources are possible, producing a CS final product having varied characteristics and not well identified profile, influencing oral absorption and activity. Finally, different extraction and purification processes may introduce further modifications of the CS structural characteristics and properties and may lead to extracts having a variable grade of purity, limited biological effects, presence of contaminants causing problems of safety and reproducibility along with not surely identified origin. These aspects pose a serious problem for the final consumers of the pharmaceutical or nutraceutical products mainly related to the traceability of CS and to the declaration of the real origin of the active ingredient and its content. In this review, specific, sensitive and validated analytical quality controls such as electrophoresis, eHPLC (enzymatic HPLC) and HPSEC (high-performance size-exclusion chromatography) able to assure CS quality and origin are illustrated and discussed.
Keywords: chondroitin sulfate; food supplements; glycosaminoglycans; nutraceuticals; osteoarthritis.
Conflict of interest statement
The author declares no conflict of interest.
Figures







Similar articles
-
Analytical aspects of pharmaceutical grade chondroitin sulfates.J Pharm Sci. 2007 Dec;96(12):3168-80. doi: 10.1002/jps.20997. J Pharm Sci. 2007. PMID: 17630645 Review.
-
Quality of different chondroitin sulfate preparations in relation to their therapeutic activity.J Pharm Pharmacol. 2009 Oct;61(10):1271-80. doi: 10.1211/jpp/61.10.0002. J Pharm Pharmacol. 2009. PMID: 19814858 Review.
-
Discrepancies in composition and biological effects of different formulations of chondroitin sulfate.Molecules. 2015 Mar 6;20(3):4277-89. doi: 10.3390/molecules20034277. Molecules. 2015. PMID: 25756648 Free PMC article. Review.
-
Structural definition of terrestrial chondroitin sulfate of various origin and repeatability of the production process.J Pharm Biomed Anal. 2021 Feb 20;195:113826. doi: 10.1016/j.jpba.2020.113826. Epub 2020 Dec 7. J Pharm Biomed Anal. 2021. PMID: 33358299
-
Comparative Analyses of Pharmaceuticals or Food Supplements Containing Chondroitin Sulfate: Are Their Bioactivities Equivalent?Adv Ther. 2019 Nov;36(11):3221-3237. doi: 10.1007/s12325-019-01064-8. Epub 2019 Sep 7. Adv Ther. 2019. PMID: 31494830 Free PMC article.
Cited by
-
Cryogel Scaffolds for Tissue-Engineering: Advances and Challenges for Effective Bone and Cartilage Regeneration.Gels. 2023 Dec 14;9(12):979. doi: 10.3390/gels9120979. Gels. 2023. PMID: 38131965 Free PMC article. Review.
-
Can Medical Cannabis Therapies be Cost-Effective in the Non-Surgical Management of Chronic Knee Pain?Clin Med Insights Arthritis Musculoskelet Disord. 2021 Mar 16;14:11795441211002492. doi: 10.1177/11795441211002492. eCollection 2021. Clin Med Insights Arthritis Musculoskelet Disord. 2021. PMID: 33795939 Free PMC article.
-
Galactosaminoglycans: Medical Applications and Drawbacks.Molecules. 2019 Aug 1;24(15):2803. doi: 10.3390/molecules24152803. Molecules. 2019. PMID: 31374852 Free PMC article. Review.
-
A Concise Review of Extraction and Characterization of Chondroitin Sulphate from Fish and Fish Wastes for Pharmacological Application.Curr Issues Mol Biol. 2022 Aug 28;44(9):3905-3922. doi: 10.3390/cimb44090268. Curr Issues Mol Biol. 2022. PMID: 36135180 Free PMC article. Review.
-
Sustainable and robust biomass-based binder for silicon anodes in lithium-ion batteries: cross-linked sodium alginate and chondroitin sulfate.Sci Technol Adv Mater. 2025 Jun 30;26(1):2523243. doi: 10.1080/14686996.2025.2523243. eCollection 2025. Sci Technol Adv Mater. 2025. PMID: 40703911 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

