Ligand-Based Stability Changes in Duplex DNA Measured with a Microscale Electrochemical Platform
- PMID: 31013753
- PMCID: PMC6628196
- DOI: 10.3390/bios9020054
Ligand-Based Stability Changes in Duplex DNA Measured with a Microscale Electrochemical Platform
Abstract
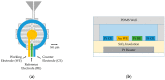
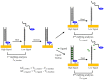
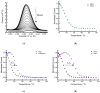
Development of technologies for rapid screening of DNA secondary structure thermal stability and the effects on stability for binding of small molecule drugs is important to the drug discovery process. In this report, we describe the capabilities of an electrochemical, microdevice-based approach for determining the melting temperatures (Tm) of electrode-bound duplex DNA structures. We also highlight new features of the technology that are compatible with array development and adaptation for high-throughput screening. As a foundational study to exhibit device performance and capabilities, melting-curve analyses were performed on 12-mer DNA duplexes in the presence/absence of two binding ligands: diminazene aceturate (DMZ) and proflavine. By measuring electrochemical current as a function of temperature, our measurement platform has the ability to determine the effect of binding ligands on Tm values with high signal-to-noise ratios and good reproducibility. We also demonstrate that heating our three-electrode cell with either an embedded microheater or a thermoelectric module produces similar results. The ΔTm values we report show the stabilizing ability of DMZ and proflavine when bound to duplex DNA structures. These initial proof-of-concept studies highlight the operating characteristics of the microdevice platform and the potential for future application toward other immobilized samples.
Keywords: DNA; electrochemical sensor; ligand-based stabilization; melting profiles; microfabrication; microheater; rapid temperature control; square wave voltammetry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
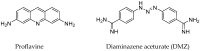

References
-
- Kohn K.W. Beyond DNA cross-linking: History and prospects of DNA-targeted cancer treatment--fifteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1996;56:5533–5546. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

