Synthesis and Luminescence of Optical Memory Active Tetramethylammonium Cyanocuprate(I) 3D Networks
- PMID: 31013868
- PMCID: PMC6514951
- DOI: 10.3390/ma12081211
Synthesis and Luminescence of Optical Memory Active Tetramethylammonium Cyanocuprate(I) 3D Networks
Abstract
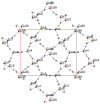
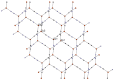
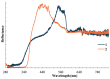
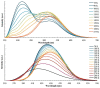
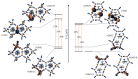
The structures of three tetramethylammonium cyanocuprate(I) 3D networks [NMe4]2[Cu(CN)2]2•0.25H2O (1), [NMe4][Cu3(CN)4] (2), and [NMe4][Cu2(CN)3] (3), (Me4N = tetramethylammonium), and the photophysics of 1 and 2 are reported. These complexes are prepared by combining aqueous solutions of the simple salts tetramethylammonium chloride and potassium dicyanocuprate. Single-crystal X-ray diffraction analysis of complex 1 reveals {Cu2(CN)2(μ2-CN)4} rhomboids crosslinked by cyano ligands and D3h {Cu(CN)3} metal clusters into a 3D coordination polymer, while 2 features independent 2D layers of fused hexagonal {Cu8(CN)8} rings where two Cu(I) centers reside in a linear C∞v coordination sphere. Metallophilic interactions are observed in 1 as close Cu⋯Cu distances, but are noticeably absent in 2. Complex 3 is a simple honeycomb sheet composed of trigonal planar Cu(I) centers with no Cu…Cu interactions. Temperature and time-dependent luminescence of 1 and 2 have been performed between 298 K and 78 K and demonstrate that 1 is a dual singlet/triplet emitter at low temperatures while 2 is a triplet-only emitter. DFT and TD-DFT calculations were used to help interpret the experimental findings. Optical memory experiments show that 1 and 2 are both optical memory active. These complexes undergo a reduction of emission intensity upon laser irradiation at 255 nm although this loss is much faster in 2. The loss of emission intensity is reversible in both cases by applying heat to the sample. We propose a light-induced electron transfer mechanism for the optical memory behavior observed.
Keywords: charge transfer; copper cyanide; crystallography; luminescence; optical memory.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Grifasi F., Chierotti M.R., Garino C., Gobetto R., Priola E., Diana E., Turci F. Solvent-Free Synthesis of Luminescent Copper(I) Coordination Polymers with Thiourea Derivatives. Cryst. Growth Des. 2015;15:2929–2939. doi: 10.1021/acs.cgd.5b00352. - DOI
-
- Safko J.P., Kuperstock J.E., McCullough S.M., Noviello A.M., Li X., Killarney J.P., Murphy C., Patterson H.H., Bayse C.A., Pike R.D. Network Formation and Photoluminescence in Copper(I) Halide Complexes with Substituted Piperazine Ligands. Dalton Trans. 2012;41:11663–11674. doi: 10.1039/c2dt31241g. - DOI - PubMed
-
- Mi J.L., Murray C.A., Tronic T.A., Dekrafft K.E., Ley A.N., DeButts J.C., Pike R.D., Lu H., Patterson H.H. Copper(I) Cyanide Networks: Synthesis, Structure, and Luminescence Behavior. Part 2. Piperazine Ligands and Hexamethylenetetramine. Inorg. Chem. 2008;47:6931–6947. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

