Snake Venom Hemotoxic Enzymes: Biochemical Comparison between Crotalus Species from Central Mexico
- PMID: 31014025
- PMCID: PMC6514926
- DOI: 10.3390/molecules24081489
Snake Venom Hemotoxic Enzymes: Biochemical Comparison between Crotalus Species from Central Mexico
Abstract
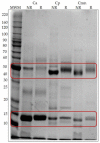
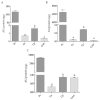
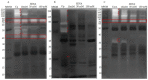
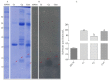
Snakebite envenoming is a serious medical problem in different areas of the world. In Latin America, the major prevalence is due to snakes of the family Viperidae, where rattlesnakes (Crotalus) are included. They produce hemotoxic venom which causes bleeding, tissue degradation and necrosis. Each venom has several enzymatic activities, producing different effects in the envenoming, doing its clinical effects difficult to study. Comparison between venom molecules is also difficult when different techniques are used, and therefore, their identification/characterization using the same methodology is necessary. In this work, a general biochemical characterization in snake venom of serine proteases (SVSP), phospholipases A2 (PLA2), metalloproteases (SVMP) and hyaluronidases (SVH) of Crotalus aquilus (Ca), Crotalus polystictus (Cp) and Crotalus molossus nigrescens (Cmn) was done. Differences in protein pattern, enzyme content and enzymatic activities were observed. All the venoms showed high PLA2 activity, high molecular weight SVSP, and a wide variety of SVMP and SVH forms. Ca and Cp showed the highest enzymatic activities of SVMP and SVSP trypsin-like and chymotrypsin-like, whereas Cmn showed the highest SVH and similar PLA2 activity with Ca. All the venoms showed peptides with similar molecular weight to crotamine-like myotoxins. No previous biochemical characterization of C. aquilus has been reported and there are no previous analyses that include these four protein families in these Crotalus venoms.
Keywords: Crotalus; hyaluronidases; metalloproteases; phospholipases A2; serine proteases; snake venom.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A Review of Rattlesnake Venoms.Toxins (Basel). 2023 Dec 19;16(1):2. doi: 10.3390/toxins16010002. Toxins (Basel). 2023. PMID: 38276526 Free PMC article. Review.
-
Ontogenetic Change in the Venom of Mexican Black-Tailed Rattlesnakes (Crotalus molossus nigrescens).Toxins (Basel). 2018 Dec 1;10(12):501. doi: 10.3390/toxins10120501. Toxins (Basel). 2018. PMID: 30513722 Free PMC article.
-
Hybridization between Crotalus aquilus and Crotalus polystictus Species: A Comparison of Their Venom Toxicity and Enzymatic Activities.Biology (Basel). 2022 Apr 26;11(5):661. doi: 10.3390/biology11050661. Biology (Basel). 2022. PMID: 35625389 Free PMC article.
-
Montane Rattlesnakes in México: Venoms of Crotalus tancitarensis and Related Species within the Crotalus intermedius Group.Toxins (Basel). 2023 Jan 13;15(1):72. doi: 10.3390/toxins15010072. Toxins (Basel). 2023. PMID: 36668891 Free PMC article.
-
Amplification of Snake Venom Toxicity by Endogenous Signaling Pathways.Toxins (Basel). 2020 Jan 22;12(2):68. doi: 10.3390/toxins12020068. Toxins (Basel). 2020. PMID: 31979014 Free PMC article. Review.
Cited by
-
A Review of Rattlesnake Venoms.Toxins (Basel). 2023 Dec 19;16(1):2. doi: 10.3390/toxins16010002. Toxins (Basel). 2023. PMID: 38276526 Free PMC article. Review.
-
Insight into the Toxicological and Pathophysiological Effects of Moroccan Vipers' Venom: Assessing the Efficacy of Commercial Antivenom for Neutralization.Trop Med Infect Dis. 2023 May 31;8(6):302. doi: 10.3390/tropicalmed8060302. Trop Med Infect Dis. 2023. PMID: 37368720 Free PMC article.
-
A Meta-Analysis of the Protein Components in Rattlesnake Venom.Toxins (Basel). 2021 May 23;13(6):372. doi: 10.3390/toxins13060372. Toxins (Basel). 2021. PMID: 34071038 Free PMC article.
-
Intraspecific Differences in the Venom of Crotalus durissus cumanensis from Colombia.Toxins (Basel). 2022 Aug 2;14(8):532. doi: 10.3390/toxins14080532. Toxins (Basel). 2022. PMID: 36006194 Free PMC article.
-
Application of an Extracellular Matrix-Mimicking Fluorescent Polymer for the Detection of Proteolytic Venom Toxins.Toxins (Basel). 2023 Apr 18;15(4):294. doi: 10.3390/toxins15040294. Toxins (Basel). 2023. PMID: 37104232 Free PMC article.
References
-
- Kasturiratne A., Wickremasinghe A.R., De Silva N., Gunawardena N.K., Pathmeswaran A., Premaratna R., Savioli L., Lalloo D.G., De Silva H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:1591–1604. doi: 10.1371/journal.pmed.0050218. - DOI - PMC - PubMed
-
- WHO . Progress in the Characterization of Venoms and Standardization of Antivenoms. World Health Organization; Geneva, Switzerland: 1981. pp. 1–44. WHO Offset Publ. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

