The Histone Deacetylase Inhibitor Suberoylanilide Hydroxamic Acid (SAHA) Restores Cardiomyocyte Contractility in a Rat Model of Early Diabetes
- PMID: 31014028
- PMCID: PMC6514644
- DOI: 10.3390/ijms20081873
The Histone Deacetylase Inhibitor Suberoylanilide Hydroxamic Acid (SAHA) Restores Cardiomyocyte Contractility in a Rat Model of Early Diabetes
Abstract
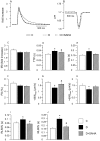
In early diabetes, hyperglycemia and the associated metabolic dysregulation promote early changes in the functional properties of cardiomyocytes, progressively leading to the appearance of the diabetic cardiomyopathy phenotype. Recently, the interplay between histone acetyltransferases (HAT) and histone deacetylases (HDAC) has emerged as a crucial factor in the development of cardiac disorders. The present study evaluates whether HDAC inhibition can prevent the development of cardiomyocyte contractile dysfunction induced by a short period of hyperglycemia, with focus on the potential underlying mechanisms. Cell contractility and calcium dynamics were measured in unloaded ventricular myocytes isolated from the heart of control and diabetic rats. Cardiomyocytes were either untreated or exposed to the pan-HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) for 90 min. Then, a fraction of each group of cells was used to evaluate the expression levels of proteins involved in the excitation-contraction coupling, and the cardiomyocyte metabolic activity, ATP content, and reactive oxygen species levels. SAHA treatment was able to counteract the initial functional derangement in cardiomyocytes by reducing cell oxidative damage. These findings suggest that early HDAC inhibition could be a promising adjuvant approach for preventing diabetes-induced cardiomyocyte oxidative damage, which triggers the pro-inflammatory signal cascade, mitochondrial damage, and ventricular dysfunction.
Keywords: HDAC inhibition; calcium transients; cardiomyocyte mechanics; cell oxidative stress; diabetes.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Zhang L., Du J., Yano N., Wang H., Zhao Y.T., Dubielecka-Szczerba P., Zhuang S., Chin E.Y., Qin G., Zhao T.C. Sodium butyrate protects against high fat diet-induced cardiac dysfunction and metabolic disorders in type II diabetic mice. J. Cell Biochem. 2017;118:2395–2408. doi: 10.1002/jcb.25902. - DOI - PMC - PubMed
-
- Savi M., Bocchi L., Sala R., Frati C., Lagrasta C., Madeddu D., Falco A., Pollino S., Bresciani L., Miragoli M., et al. Parenchymal and Stromal Cells Contribute to Pro-Inflammatory Myocardial Environment at Early Stages of Diabetes: Protective Role of Resveratrol. Nutrients. 2016;8:729. doi: 10.3390/nu8110729. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

