Influenza Hemagglutinin and Neuraminidase: Yin⁻Yang Proteins Coevolving to Thwart Immunity
- PMID: 31014029
- PMCID: PMC6520700
- DOI: 10.3390/v11040346
Influenza Hemagglutinin and Neuraminidase: Yin⁻Yang Proteins Coevolving to Thwart Immunity
Abstract
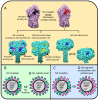
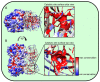
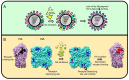
Influenza A virions possess two surface glycoproteins-the hemagglutinin (HA) and neuraminidase (NA)-which exert opposite functions. HA attaches virions to cells by binding to terminal sialic acid residues on glycoproteins/glycolipids to initiate the infectious cycle, while NA cleaves terminal sialic acids, releasing virions to complete the infectious cycle. Antibodies specific for HA or NA can protect experimental animals from IAV pathogenesis and drive antigenic variation in their target epitopes that impairs vaccine effectiveness in humans. Here, we review progress in understanding HA/NA co-evolution as each acquires epistatic mutations to restore viral fitness to mutants selected in the other protein by host innate or adaptive immune pressure. We also discuss recent exciting findings that antibodies to HA can function in vivo by blocking NA enzyme activity to prevent nascent virion release and enhance Fc receptor-based activation of innate immune cells.
Keywords: Influenza A virus; antigenic drift; hemagglutinin; neuraminidase; viral evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Eichelberger M.C., Wan H. Influenza neuraminidase as a vaccine antigen. Curr. Top. Microbiol. Immunol. 2015;386:275–299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

