The effectiveness of cinacalcet: a randomized, open label study in chronic hemodialysis patients with severe secondary hyperparathyroidism
- PMID: 31014177
- PMCID: PMC6493313
- DOI: 10.1080/0886022X.2018.1562356
The effectiveness of cinacalcet: a randomized, open label study in chronic hemodialysis patients with severe secondary hyperparathyroidism
Abstract
Background: Secondary hyperparathyroidism (SHPT) is associated with high incidences of cardiovascular disease, bone fracture, and mortality. This study was conducted to demonstrate the effectiveness of cinacalcet treatment on chronic kidney disease-mineral bone disorder (CKD-MBD) markers in chronic hemodialysis patients with severe SHPT.
Methods: In phase 1, 30 adult HD patients were randomized to cinacalcet or control groups for 12 weeks to explore the achievement of >30% reduction of iPTH. In phase 2, 45 patients were participated to further explore the effect of cinacalcet on CKD-MBD parameters for 24-week follow up and 12 additional weeks after cinacalcet discontinuation.
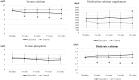
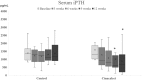
Results: In phase 1, the baseline serum iPTH levels were not different [1374 (955, 1639) pg/mL in the control group vs. 1191 (1005, 1884) pg/mL in the cinacalcet group], the percentage of patients achieving iPTH target were significantly higher in the treatment group [80% vs. 13%, p = .001]. In phase 2, the significant reductions of iPTH, FGF-23, tartrate-resistant acid phosphatase 5b, and slightly decreased size of parathyroid gland and stabilized vascular calcification were observed at 24-week follow up and markedly rebounded after discontinuation of cinacalcet.
Conclusions: The effectiveness of cinacalcet were still obviously demonstrated even in chronic HD patients with severe SHPT. In addition, the improvements of bone markers and FGF-23, and stabilization of vascular calcification were observed. Therefore, cinacalcet can provide salutary effects on CKD-MBD in severe SHPT and might be an initially effective PTH-lowering therapy prior to surgical parathyroidectomy as well as an alternative treatment in the patients unsuitable for surgery.
Clinical trial registration: ClinicalTrials.gov: NCT02056730. Date of registration: February 4, 2014.
Keywords: Cinacalcet; chronic hemodialysis; severe secondary hyperparathyroidism.
Figures
Similar articles
-
Effect of Cinacalcet and Vitamin D Analogs on Fibroblast Growth Factor-23 during the Treatment of Secondary Hyperparathyroidism.Clin J Am Soc Nephrol. 2015 Jun 5;10(6):1021-30. doi: 10.2215/CJN.03270314. Epub 2015 Apr 14. Clin J Am Soc Nephrol. 2015. PMID: 25873267 Free PMC article. Clinical Trial.
-
The impact of cinacalcet in the mineral metabolism markers of patients on dialysis with severe secondary hyperparathyroidism.J Bras Nefrol. 2019 Jul 18;41(3):336-344. doi: 10.1590/2175-8239-JBN-2018-0219. J Bras Nefrol. 2019. PMID: 31419274 Free PMC article.
-
Long-Term Efficacy and Safety of Upacicalcet in Japanese Hemodialysis Patients with Secondary Hyperparathyroidism: Open-Label 52-Week Study.Am J Nephrol. 2025;56(1):70-84. doi: 10.1159/000541493. Epub 2024 Sep 19. Am J Nephrol. 2025. PMID: 39299219 Free PMC article. Clinical Trial.
-
Comparative Effectiveness of Calcimimetic Agents for Secondary Hyperparathyroidism in Adults: A Systematic Review and Network Meta-analysis.Am J Kidney Dis. 2020 Sep;76(3):321-330. doi: 10.1053/j.ajkd.2020.02.439. Epub 2020 May 28. Am J Kidney Dis. 2020. PMID: 32475604
-
Cinacalcet HCl: a novel treatment for secondary hyperparathyroidism caused by chronic kidney disease.J Ren Nutr. 2006 Jul;16(3):253-8. doi: 10.1053/j.jrn.2006.04.010. J Ren Nutr. 2006. PMID: 16825031 Review.
Cited by
-
Fracture Risk in Chronic Kidney Disease: Addressing an Overlooked Complication.Metabolites. 2025 Jul 7;15(7):460. doi: 10.3390/metabo15070460. Metabolites. 2025. PMID: 40710560 Free PMC article. Review.
-
Comparative efficacy of sodium thiosulfate, bisphosphonates, and cinacalcet for the treatment of vascular calcification in patients with haemodialysis: a systematic review and network meta-analysis.BMC Nephrol. 2024 Jan 22;25(1):26. doi: 10.1186/s12882-024-03460-x. BMC Nephrol. 2024. PMID: 38254024 Free PMC article.
-
Risk factors for calciphylaxis in Chinese hemodialysis patients: a matched case-control study.Ren Fail. 2021 Dec;43(1):406-416. doi: 10.1080/0886022X.2021.1884094. Ren Fail. 2021. PMID: 33641601 Free PMC article.
-
Efficacy and Safety of Evocalcet Evaluated by Dialysate Calcium Concentration in Patients with Secondary Hyperparathyroidism Undergoing Hemodialysis.Int J Nephrol Renovasc Dis. 2020 May 12;13:97-106. doi: 10.2147/IJNRD.S243210. eCollection 2020. Int J Nephrol Renovasc Dis. 2020. PMID: 32494184 Free PMC article.
-
Cinacalcet use in pediatric chronic kidney disease. A survey study.Saudi Med J. 2020 May;41(5):479-484. doi: 10.15537/smj.2020.5.25072. Saudi Med J. 2020. PMID: 32373914 Free PMC article.
References
-
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group KDIGO clinical practice guideline for the diagnosis. Evaluation, prevention, and treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD–MBD). Kidney Int. 2009;76:S1–S130. - PubMed
-
- Bouma-de Krijger A, Bots ML, Vervloet MG, et al. . Time-averaged level of fibroblast growth factor-23 and clinical events in chronic kidney disease. Nephrol Dial Transplant. 2014;29:88–97. - PubMed
-
- Cernaro V, Santoro D, Lucisano S, et al. . The future of phosphate binders: a perspective on novel therapeutics. Expert Opin Investig Drugs. 2014;23:1459–1463. - PubMed
-
- Djukanović L, Dimković N, Marinković J, et al. . Association between hemodialysis patient outcomes and compliance with KDOQI and KDIGO targets for mineral and bone metabolism. Nephron. 2016;132:168–174. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
