A preliminary investigation of circulating extracellular vesicles and biomarker discovery associated with treatment response in head and neck squamous cell carcinoma
- PMID: 31014274
- PMCID: PMC6480898
- DOI: 10.1186/s12885-019-5565-9
A preliminary investigation of circulating extracellular vesicles and biomarker discovery associated with treatment response in head and neck squamous cell carcinoma
Abstract
Background: There is a paucity of plasma-based biomarkers that prospectively segregate the outcome of patients with head and neck squamous-cell carcinoma (HNSCC) treated with chemoradiation therapy (CRT). Plasma extracellular vesicles (EVs) might be an alternative source for discovery of new specific markers present in patients with HNSCC, which could help to re-direct patients to appropriate curative therapies without delay.
Methods: In order to identify new markers in plasma compartments, Cholerae toxin B chain (CTB) and Annexin V (AV) were used to isolate EVs from pooled plasma samples from patients with locally advanced HNSCC who responded (CR, n = 6) or presented incomplete response (NR, n = 6) to CRT. The crude plasma and EVs cargo were screened by antibody array.
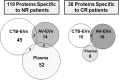
Results: Of the 370 polypeptides detected, 119 proteins were specific to NR patients while 38 were exclusive of the CR subjects. The Gene Set Enrichment Analysis (GSEA) and Search Tool for the Retrieval of Interacting Genes (STRING) database analysis indicated that the content of circulating plasma EVs might have a relevant function for the tumor intercellular communication in the HNSCC patients.
Conclusion: This study provides a list of potential markers present in plasma compartments that might contribute to the development of tools for prediction and assessment of CRT response and potentially guide therapeutic decisions in this context.
Keywords: Biomarker discovery; Chemoradiation therapy; Extracellular vesicles; HNSCC; Head and neck squamous cell carcinoma; Treatment response.
Conflict of interest statement
Ethics approval and consent to participate
Written informed consent was obtained from all HNSCC patients. This study was approved by the institution ethics committees (CEP-UNIFESP: 1610/2016 and CEP-HCBarretos: 231/2009).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Iyer NG, Tan DS, Tan VK, et al. Randomized trial comparing surgery and adjuvant radiotherapy versus concurrent chemoradiotherapy in patients with advanced, nonmetastatic squamous cell carcinoma of the head and neck: 10-year update and subset analysis. Cancer. 2015;121:1599–1607. doi: 10.1002/cncr.29251. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

