The French glioblastoma biobank (FGB): a national clinicobiological database
- PMID: 31014363
- PMCID: PMC6480741
- DOI: 10.1186/s12967-019-1859-6
The French glioblastoma biobank (FGB): a national clinicobiological database
Erratum in
-
Correction to: The French glioblastoma biobank (FGB): a national clinicobiological database.J Transl Med. 2020 Apr 30;18(1):181. doi: 10.1186/s12967-020-02345-5. J Transl Med. 2020. PMID: 32354338 Free PMC article.
Abstract
Background: Glioblastomas (GB) are the most common and lethal primary brain tumors. Significant progress has been made toward identifying potential risk factors for GB and diagnostic and prognostic biomarkers. However, the current standard of care for newly diagnosed GB, the Stupp protocol, has remained unchanged for over a decade. Large-scale translational programs based on a large clinicobiological database are required to improve our understanding of GB biology, potentially facilitating the development of personalized and specifically targeted therapies. With this goal in mind, a well-annotated clinicobiological database housing data and samples from GB patients has been set up in France: the French GB biobank (FGB).
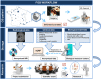
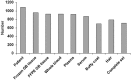
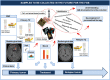
Methods: The biobank contains data and samples from adult GB patients from 24 centers in France providing written informed consent. Clinical and biomaterial data are stored in anonymized certified electronic case report forms. Biological samples (including frozen and formalin-fixed paraffin-embedded tumor tissues, blood samples, and hair) are conserved in certified biological resource centers or tumor tissue banks at each participating center.
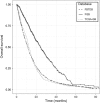
Results: Clinical data and biological materials have been collected for 1087 GB patients. A complete set of samples (tumor, blood and hair) is available for 66%, and at least one frozen tumor sample is available for 88% of the GB patients.
Conclusions: This large biobank is unique in Europe and can support the large-scale translational projects required to improve GB care. Additional biological materials, such as peritumoral brain zone and fecal samples, will be collected in the future, to respond to research needs.
Keywords: Biobank; Biological materials; Clinical data; Database; Glioblastoma.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- Darlix A, Zouaoui S, Rigau V, Bessaoud F, Figarella-Branger D, Mathieu-Daudé H, et al. Epidemiology for primary brain tumors: a nationwide population-based study. J Neurooncol. 2017;131:525–546. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

