Effects of different modalities of inspiratory muscle training as an add-on to conventional treatment of patients with chronic obstructive pulmonary disease (COPD): study protocol for a randomized controlled trial
- PMID: 31014365
- PMCID: PMC6480485
- DOI: 10.1186/s13063-019-3271-1
Effects of different modalities of inspiratory muscle training as an add-on to conventional treatment of patients with chronic obstructive pulmonary disease (COPD): study protocol for a randomized controlled trial
Abstract
Background: Chronic obstructive pulmonary disease (COPD) leads to peripheral and respiratory muscle dysfunctions. Nowadays, inspiratory muscle training can be geared toward strength or endurance gains. This study aims to investigate the effects of an inspiratory muscle training (IMT) protocol using different therapeutic modalities to be implemented in pulmonary rehabilitation programs. The effects of IMT on exercise capacity were considered as the primary endpoint, and the effects of IMT on inspiratory muscle function, health-related quality of life, and daily physical activity level were considered as the secondary outcomes.
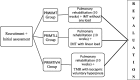
Methods: This study is a blinded-investigator randomized controlled clinical trial. Sixty subjects will be randomly allocated into three groups: (1) pulmonary rehabilitation (PR) associated with inspiratory muscle training without any load (PRWIMT), (2) PR associated with inspiratory muscle training with a linear load (PRIMTLL), and (3) PR associated with inspiratory muscle training with isocapnic voluntary hyperpnea (PRIMTIVH). The protocol will be performed 5 days a week (3 days with supervision) for 10 weeks. The study will assess anthropometric data, lung function, respiratory muscle strength, and functional capacity by the Incremental Shuttle Walking Test and the Six-Minute Walk Test, lung volumes during the submaximal endurance test, peripheral muscle strength of the upper and lower limbs, dyspnea, and quality of life related to health, before and after the training protocol. Normality will be tested using the Kolmogorov-Smirnov test, and variables will be compared by two-way analysis of variance. The significance level was set at p < 0.05. Ethics approval was obtained from the Institutional Ethics Committee in Research (1.663.411). The study results will be disseminated through presentation at specific scientific conferences and publication in peer-reviewed journals.
Discussion: The different IMT protocols used in our study will be able to guide respiratory therapists to understand and to include in conventional PR programs the most effective respiratory muscle training type in subjects with COPD.
Trial registration: Brazilian Clinical Trials Registry, RBR-94v6kd . Registered on 11 March 2017.
Keywords: COPD; Pulmonary rehabilitation; Respiratory muscle training; Training.
Conflict of interest statement
Ethics approval and consent to participate
This study was registered in the Brazilian Clinical Trials Registry (REQ-4073) and was approved by the Institutional Ethics Committee in Research (1.663.411) of the University Hospital Onofre Lopes. All subjects will receive information regarding their participation in the study and will sign the free and informed consent form in accordance with the resolution 466/12 of the National Health Council and the 1975 Declaration of Helsinki [56].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. Available from: http://goldcopd.org. Accessed 21 Mar 2019.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials