Particle toxicology and health - where are we?
- PMID: 31014371
- PMCID: PMC6480662
- DOI: 10.1186/s12989-019-0302-8
Particle toxicology and health - where are we?
Erratum in
-
Correction to: Particle toxicology and health - where are we?Part Fibre Toxicol. 2019 Jun 27;16(1):26. doi: 10.1186/s12989-019-0308-2. Part Fibre Toxicol. 2019. PMID: 31248442 Free PMC article.
Abstract
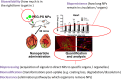
Background: Particles and fibres affect human health as a function of their properties such as chemical composition, size and shape but also depending on complex interactions in an organism that occur at various levels between particle uptake and target organ responses. While particulate pollution is one of the leading contributors to the global burden of disease, particles are also increasingly used for medical purposes. Over the past decades we have gained considerable experience in how particle properties and particle-bio interactions are linked to human health. This insight is useful for improved risk management in the case of unwanted health effects but also for developing novel medical therapies. The concepts that help us better understand particles' and fibres' risks include the fate of particles in the body; exposure, dosimetry and dose-metrics and the 5 Bs: bioavailability, biopersistence, bioprocessing, biomodification and bioclearance of (nano)particles. This includes the role of the biomolecule corona, immunity and systemic responses, non-specific effects in the lungs and other body parts, particle effects and the developing body, and the link from the natural environment to human health. The importance of these different concepts for the human health risk depends not only on the properties of the particles and fibres, but is also strongly influenced by production, use and disposal scenarios.
Conclusions: Lessons learned from the past can prove helpful for the future of the field, notably for understanding novel particles and fibres and for defining appropriate risk management and governance approaches.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable (this is a review article).
Consent for publication
Not applicable (this is a review article).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- GBD 2015 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:P1659–P1724. doi: 10.1016/S0140-6736(16)31679-8. - DOI - PMC - PubMed
-
- Kuempel ED, Jaurand MC, Møller P, Morimoto Y, Kobayashi N, Pinkerton KE, Sargent LM, Vermeulen RCH, Fubini B, Kane AB. Evaluating the mechanistic evidence and key data gaps in assessing the potential carcinogenicity of carbon nanotubes and nanofibers in humans. Crit Rev Toxicol. 2017;47(1):1–58. - PMC - PubMed
-
- Bouwmeester H, Lynch I, Marvin HJ, Dawson KA, Berges M, Braguer D, Byrne HJ, Casey A, Chambers G, Clift MJ, Elia G, Fernandes TF, Fjellsbø LB, Hatto P, Juillerat L, Klein C, Kreyling WG, Nickel C, Riediker M, Stone V. Minimal analytical characterization of engineered nanomaterials needed for hazard assessment in biological matrices. Nanotoxicology. 2011;5(1):1–11. doi: 10.3109/17435391003775266. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

