Platelet-rich plasma versus lidocaine as tenotomy adjuvants in people with elbow epicondylopathy: a randomized controlled trial
- PMID: 31014382
- PMCID: PMC6480601
- DOI: 10.1186/s13018-019-1153-6
Platelet-rich plasma versus lidocaine as tenotomy adjuvants in people with elbow epicondylopathy: a randomized controlled trial
Abstract
Objectives: To determine the efficacy of platelet-rich plasma (PRP) compared to lidocaine as a tenotomy adjuvant for people with elbow tendinopathy.
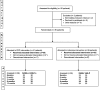
Methods: Our study was a parallel-group, double-blind, randomized trial involving 71 patients with recalcitrant elbow tendinopathy who received two sessions of ultrasound-guided tenotomy with either PRP or lidocaine in a tertiary public hospital. The primary end point was the percentage of patients with an improvement exceeding 25% reduction in disability (Spanish version of the Disabilities of the Arm, Shoulder and Hand questionnaires-DASH-E) at 6 and 12 months; the secondary outcome was the percentage of patients exceeding 25% reduction in pain (VAS-P).
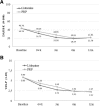
Results: There was no evidence of significant differences in the proportion of patients who experienced clinically relevant improvements. After 6 months, 18 patients (78.59%) in the lidocaine group and 19 patients (73.08%) in the PRP group showed improved function above 25% (unadjusted odds ratio, 0.90; 95% confidence interval [CI], 0.90 (0.17 to 4.60)); 21 patients (72.21%) in the lidocaine group versus 22 patients (84.62%) in the PRP group achieved more than 25% pain reduction (unadjusted odds ratio, 0.48; 95% CI, 0.10 to 2.37). After 12 months, 17 patients (70.83%) in the lidocaine group versus 19 patients (76%) in the PRP group had improved function (unadjusted odds ratio, 0.71; 95% CI, 0.13 to 3.84), and 19 patients (76%) in the lidocaine group versus 20 patients (90.91%) in the PRP group had improved pain above 25% (unadjusted odds ratio, 0.35; 95% CI, 0.06 to 2.51). Hypercholesterolemia and baseline vascularization influenced outcomes. There were no differences between groups in the adjusted odds ratios.
Conclusion: PRP results in similar improvements to those obtained with lidocaine. Selecting patients according to their pretreatment status can improve treatment efficacy.
Trial registration: NCT01945528 , EudraCT 2013-000478-32. Registered 18 August 2013, enrolment of the first participant 10 March 2014.
Keywords: Elbow tendinopathy; Epicondylitis; Function; Lateral; Medial; Pain; Platelet-rich plasma; Randomized controlled study; Sonography; Tenotomy.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Hospital Universitario Cruces (No. CEIC 13/04). All enrolled patients provided written informed consent. The study protocol was registered at
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
All participants gave their written consent for publication
Competing interests
The authors declare no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

