Gsα deficiency in the dorsomedial hypothalamus leads to obesity, hyperphagia, and reduced thermogenesis associated with impaired leptin signaling
- PMID: 31014927
- PMCID: PMC6601467
- DOI: 10.1016/j.molmet.2019.04.005
Gsα deficiency in the dorsomedial hypothalamus leads to obesity, hyperphagia, and reduced thermogenesis associated with impaired leptin signaling
Abstract
Objective: Gsα couples multiple receptors, including the melanocortin 4 receptor (MC4R), to intracellular cAMP generation. Germline inactivating Gsα mutations lead to obesity in humans and mice. Mice with brain-specific Gsα deficiency also develop obesity with reduced energy expenditure and locomotor activity, and impaired adaptive thermogenesis, but the underlying mechanisms remain unclear.
Methods: We created mice (DMHGsKO) with Gsα deficiency limited to the dorsomedial hypothalamus (DMH) and examined the effects on energy balance and thermogenesis.
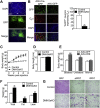
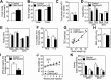
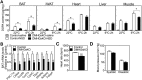
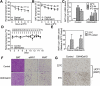
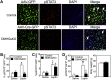
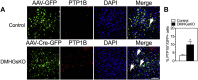
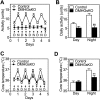
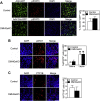
Results: DMHGsKO mice developed severe, early-onset obesity associated with hyperphagia and reduced energy expenditure and locomotor activity, along with impaired brown adipose tissue thermogenesis. Studies in mice with loss of MC4R in the DMH suggest that defective DMH MC4R/Gsα signaling contributes to abnormal energy balance but not to abnormal locomotor activity or cold-induced thermogenesis. Instead, DMHGsKO mice had impaired leptin signaling along with increased expression of the leptin signaling inhibitor protein tyrosine phosphatase 1B in the DMH, which likely contributes to the observed hyperphagia and reductions in energy expenditure, locomotor activity, and cold-induced thermogenesis.
Conclusions: DMH Gsα signaling is critical for energy balance, thermogenesis, and leptin signaling. This study provides insight into how distinct signaling pathways can interact to regulate energy homeostasis and temperature regulation.
Keywords: G protein; Hypothalamus; Obesity; Sympathetic nervous system; Thermogenesis.
Published by Elsevier GmbH.
Figures


Similar articles
-
Gsα deficiency in the dorsomedial hypothalamus underlies obesity associated with Gsα mutations.J Clin Invest. 2017 Feb 1;127(2):500-510. doi: 10.1172/JCI88622. Epub 2016 Dec 19. J Clin Invest. 2017. PMID: 27991864 Free PMC article.
-
The stimulatory G protein Gsα is required in melanocortin 4 receptor-expressing cells for normal energy balance, thermogenesis, and glucose metabolism.J Biol Chem. 2018 Jul 13;293(28):10993-11005. doi: 10.1074/jbc.RA118.003450. Epub 2018 May 24. J Biol Chem. 2018. PMID: 29794140 Free PMC article.
-
Gqα/G11α deficiency in dorsomedial hypothalamus leads to obesity resulting from decreased energy expenditure and impaired sympathetic nerve activity.Am J Physiol Endocrinol Metab. 2021 Feb 1;320(2):E270-E280. doi: 10.1152/ajpendo.00059.2020. Epub 2020 Nov 9. Am J Physiol Endocrinol Metab. 2021. PMID: 33166186 Free PMC article.
-
Role of leptin in energy expenditure: the hypothalamic perspective.Am J Physiol Regul Integr Comp Physiol. 2017 Jun 1;312(6):R938-R947. doi: 10.1152/ajpregu.00045.2016. Epub 2017 Mar 29. Am J Physiol Regul Integr Comp Physiol. 2017. PMID: 28356295 Review.
-
Dorsomedial hypothalamic NPY and energy balance control.Neuropeptides. 2012 Dec;46(6):309-14. doi: 10.1016/j.npep.2012.09.002. Epub 2012 Oct 18. Neuropeptides. 2012. PMID: 23083763 Free PMC article. Review.
Cited by
-
Secretin-dependent signals in the ventromedial hypothalamus regulate energy metabolism and bone homeostasis in mice.Nat Commun. 2024 Feb 3;15(1):1030. doi: 10.1038/s41467-024-45436-3. Nat Commun. 2024. PMID: 38310104 Free PMC article.
-
Hypothalamic GPCR Signaling Pathways in Cardiometabolic Control.Front Physiol. 2021 Jun 28;12:691226. doi: 10.3389/fphys.2021.691226. eCollection 2021. Front Physiol. 2021. PMID: 34262481 Free PMC article. Review.
-
Medial hypothalamic MC3R signalling regulates energy rheostasis in adult mice.J Physiol. 2025 Jan;603(2):379-410. doi: 10.1113/JP286699. Epub 2024 Dec 24. J Physiol. 2025. PMID: 39718394 Free PMC article.
-
Neuroanatomical dissection of the MC3R circuitry regulating energy rheostasis.bioRxiv [Preprint]. 2024 Apr 26:2024.04.22.590573. doi: 10.1101/2024.04.22.590573. bioRxiv. 2024. PMID: 38712101 Free PMC article. Preprint.
-
gnas Knockdown Induces Obesity and AHO Features in Early Zebrafish Larvae.Int J Mol Sci. 2024 Nov 26;25(23):12674. doi: 10.3390/ijms252312674. Int J Mol Sci. 2024. PMID: 39684386 Free PMC article.
References
-
- Huszar D., Lynch C.A., Fairchild-Huntress V., Dunmore J.H., Fang Q., Berkemeier L.R. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. - PubMed
-
- Farooqi I.S., Keogh J.M., Yeo G.S., Lank E.J., Cheetham T., O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. New England Journal of Medicine. 2003;348:1085–1095. - PubMed
-
- Fan W., Morrison S.F., Cao W.H., Yu P. Thermogenesis activated by central melanocortin signaling is dependent on neurons in the rostral raphe pallidus (rRpa) area. Brain Research. 2007;1179:61–619. - PubMed
-
- Butler A.A., Cone R.D. The melanocortin receptors: lessons from knockout models. Neuropeptides. 2002;36:77–84. - PubMed
-
- Voss-Andreae A., Murphy J.G., Ellacott K.L., Stuart R.C., Nillni E.A., Cone R.D. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology. 2007;148:1550–1560. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

