Network Medicine in Pathobiology
- PMID: 31014954
- PMCID: PMC6616999
- DOI: 10.1016/j.ajpath.2019.03.009
Network Medicine in Pathobiology
Abstract
The past decade has witnessed exponential growth in the generation of high-throughput human data across almost all known dimensions of biological systems. The discipline of network medicine has rapidly evolved in parallel, providing an unbiased, comprehensive biological framework through which to interrogate and integrate systematically these large-scale, multi-omic data to enhance our understanding of disease mechanisms and to design drugs that reflect a deep knowledge of molecular pathobiology. In this review, we discuss the key principles of network medicine and the human disease network and explore the latest applications of network medicine in this multi-omic era. We also highlight the current conceptual and technological challenges, which serve as exciting opportunities by which to improve and expand the network-based applications beyond the artificial boundaries of the current state of human pathobiology.
Copyright © 2019 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
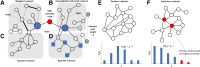
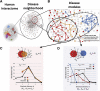

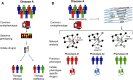


References
-
- Loscalzo J., Kohane I., Barabasi A.L. Human disease classification in the postgenomic era: a complex systems approach to human pathobiology. Mol Syst Biol. 2007;3:124. - PMC - PubMed
- Loscalzo J, Kohane I, Barabasi AL: Human disease classification in the postgenomic era: a complex systems approach to human pathobiology. Mol Syst Biol 2007, 3:124. - PMC - PubMed
-
- Loscalzo J., Barabási A.-L.S., Silverman E.K. Harvard University Press; Cambridge, Massachusetts: 2017. Network medicine: complex systems in human disease and therapeutics.
- Loscalzo J, Barabási A-Ls, Silverman EK: Network medicine: complex systems in human disease and therapeutics. Cambridge, Massachusetts: Harvard University Press, 2017.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

