Regulation of Drosophila germline stem cells
- PMID: 31014993
- PMCID: PMC6756965
- DOI: 10.1016/j.ceb.2019.03.008
Regulation of Drosophila germline stem cells
Abstract
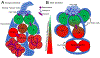
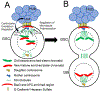
The asymmetric division of adult stem cells into one self-renewing stem cell and one differentiating cell is critical for maintaining homeostasis in many tissues. One paradigmatic model of this division is the Drosophila male and female germline stem cell, which provides two model systems not only sharing common features but also having distinct characteristics for studying asymmetric stem cell division in vivo. This asymmetric division is controlled by a combination of extrinsic signaling molecules and intrinsic factors that are either asymmetrically segregated or regulated differentially following division. In this review, we will discuss recent advances in understanding the molecular and cellular mechanisms guiding this asymmetric outcome, including extrinsic cues, intrinsic factors governing cell fate specification, and cell cycle control.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

Similar articles
-
Asymmetric division of Drosophila male germline stem cell shows asymmetric histone distribution.Science. 2012 Nov 2;338(6107):679-82. doi: 10.1126/science.1226028. Science. 2012. PMID: 23118191 Free PMC article.
-
Bam and Bgcn in Drosophila germline stem cell differentiation.Vitam Horm. 2011;87:399-416. doi: 10.1016/B978-0-12-386015-6.00038-X. Vitam Horm. 2011. PMID: 22127253 Review.
-
Fly meets yeast: checking the correct orientation of cell division.Trends Cell Biol. 2011 Sep;21(9):526-33. doi: 10.1016/j.tcb.2011.05.004. Epub 2011 Jun 24. Trends Cell Biol. 2011. PMID: 21705221 Free PMC article. Review.
-
Asymmetric Histone Inheritance in Asymmetrically Dividing Stem Cells.Trends Genet. 2020 Jan;36(1):30-43. doi: 10.1016/j.tig.2019.10.004. Epub 2019 Nov 18. Trends Genet. 2020. PMID: 31753528 Free PMC article. Review.
-
Centrosome misorientation mediates slowing of the cell cycle under limited nutrient conditions in Drosophila male germline stem cells.Mol Biol Cell. 2012 Apr;23(8):1524-32. doi: 10.1091/mbc.E11-12-0999. Epub 2012 Feb 22. Mol Biol Cell. 2012. PMID: 22357619 Free PMC article.
Cited by
-
Niche formation and function in developing tissue: studies from the Drosophila ovary.Cell Commun Signal. 2023 Jan 27;21(1):23. doi: 10.1186/s12964-022-01035-7. Cell Commun Signal. 2023. PMID: 36707894 Free PMC article. Review.
-
Communication between the stem cell niche and an adjacent differentiation niche through miRNA and EGFR signaling orchestrates exit from the stem cell state in the Drosophila ovary.PLoS Biol. 2024 Mar 21;22(3):e3002515. doi: 10.1371/journal.pbio.3002515. eCollection 2024 Mar. PLoS Biol. 2024. PMID: 38512963 Free PMC article.
-
Female-germline specific protein Sakura interacts with Otu and is crucial for germline stem cell renewal and differentiation and oogenesis.Elife. 2025 Jul 15;13:RP103828. doi: 10.7554/eLife.103828. Elife. 2025. PMID: 40663062 Free PMC article.
-
Recent insights into the microRNA and long non-coding RNA-mediated regulation of stem cell populations.3 Biotech. 2022 Oct;12(10):270. doi: 10.1007/s13205-022-03343-8. Epub 2022 Sep 10. 3 Biotech. 2022. PMID: 36101546 Free PMC article. Review.
-
Coordinating Proliferation, Polarity, and Cell Fate in the Drosophila Female Germline.Front Cell Dev Biol. 2020 Feb 4;8:19. doi: 10.3389/fcell.2020.00019. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32117961 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

