Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice
- PMID: 31015147
- PMCID: PMC6477631
- DOI: 10.1016/j.redox.2019.101192
Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice
Abstract
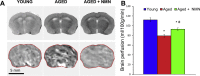
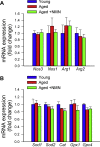
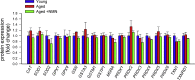
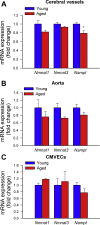
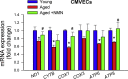
Adjustment of cerebral blood flow (CBF) to neuronal activity via neurovascular coupling (NVC) has an essential role in maintenance of healthy cognitive function. In aging increased oxidative stress and cerebromicrovascular endothelial dysfunction impair NVC, contributing to cognitive decline. There is increasing evidence showing that a decrease in NAD+ availability with age plays a critical role in a range of age-related cellular impairments but its role in impaired NVC responses remains unexplored. The present study was designed to test the hypothesis that restoring NAD+ concentration may exert beneficial effects on NVC responses in aging. To test this hypothesis 24-month-old C57BL/6 mice were treated with nicotinamide mononucleotide (NMN), a key NAD+ intermediate, for 2 weeks. NVC was assessed by measuring CBF responses (laser Doppler flowmetry) evoked by contralateral whisker stimulation. We found that NVC responses were significantly impaired in aged mice. NMN supplementation rescued NVC responses by increasing endothelial NO-mediated vasodilation, which was associated with significantly improved spatial working memory and gait coordination. These findings are paralleled by the sirtuin-dependent protective effects of NMN on mitochondrial production of reactive oxygen species and mitochondrial bioenergetics in cultured cerebromicrovascular endothelial cells derived from aged animals. Thus, a decrease in NAD+ availability contributes to age-related cerebromicrovascular dysfunction, exacerbating cognitive decline. The cerebromicrovascular protective effects of NMN highlight the preventive and therapeutic potential of NAD+ intermediates as effective interventions in patients at risk for vascular cognitive impairment (VCI).
Keywords: Endothelial dysfunction; Functional hyperemia; Microcirculation; Oxidative stress; ROS.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures












References
-
- Toth P., Tarantini S., Csiszar A., Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Heart Circ. Physiol. 2017;312:H1–H20. - PMC - PubMed
-
- Toth P., Tarantini S., Davila A., Valcarcel-Ares M.N., Tucsek Z., Varamini B., Ballabh P., Sonntag W.E., Baur J.A., Csiszar A., Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H1837–H1845. - PMC - PubMed
-
- Toth P., Tarantini S., Tucsek Z., Ashpole N.M., Sosnowska D., Gautam T., Ballabh P., Koller A., Sonntag W.E., Csiszar A., Ungvari Z.I. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H299–H308. - PMC - PubMed
-
- Zaletel M., Strucl M., Pretnar-Oblak J., Zvan B. Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity response. Funct. Neurol. 2005;20:115–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG038747/AG/NIA NIH HHS/United States
- R01 AG055395/AG/NIA NIH HHS/United States
- DP1 AG058605/AG/NIA NIH HHS/United States
- R01 DK100263/DK/NIDDK NIH HHS/United States
- R01 AG019719/AG/NIA NIH HHS/United States
- R01 NS056218/NS/NINDS NIH HHS/United States
- R01 DK098656/DK/NIDDK NIH HHS/United States
- U54 GM104938/GM/NIGMS NIH HHS/United States
- P20 GM103447/GM/NIGMS NIH HHS/United States
- T32 AG052363/AG/NIA NIH HHS/United States
- R21 DE027490/DE/NIDCR NIH HHS/United States
- R01 AG047879/AG/NIA NIH HHS/United States
- P30 AG050911/AG/NIA NIH HHS/United States
- R37 AG028730/AG/NIA NIH HHS/United States
- R01 HL132553/HL/NHLBI NIH HHS/United States
- P20 GM125528/GM/NIGMS NIH HHS/United States
- R01 AG043483/AG/NIA NIH HHS/United States
- R01 NS100782/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

