Deficiency of adenosine deaminase 2 triggers adenosine-mediated NETosis and TNF production in patients with DADA2
- PMID: 31015188
- PMCID: PMC6659253
- DOI: 10.1182/blood.2018892752
Deficiency of adenosine deaminase 2 triggers adenosine-mediated NETosis and TNF production in patients with DADA2
Abstract
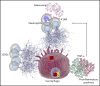
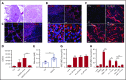
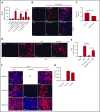
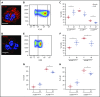
Reduction of adenosine deaminase 2 (ADA2) activity due to autosomal-recessive loss-of-function mutations in the ADA2 gene (previously known as CECR1) results in a systemic vasculitis known as deficiency of ADA2 (DADA2). Neutrophils and a subset of neutrophils known as low-density granulocytes (LDGs) have been implicated in the pathogenesis of vasculitis, at least in part, through the formation of neutrophil extracellular traps (NETs). The study objective was to determine whether neutrophils and NETs play a pathogenic role in DADA2. In vivo evidence demonstrated NETs and macrophages in affected gastrointestinal tissue from patients with DADA2. An abundance of circulating LDGs prone to spontaneous NET formation was observed during active disease in DADA2 and were significantly reduced after remission induction by anti-tumor necrosis factor (TNF) therapy. Increased circulating LDGs were identified in unaffected family members with monoallelic ADA2 mutations. Adenosine triggered NET formation, particularly in neutrophils from female patients, by engaging A1 and A3 adenosine receptors (ARs) and through reactive oxygen species- and peptidylarginine deiminase-dependent pathways. Adenosine-induced NET formation was inhibited by recombinant ADA2, A1/A3 AR antagonists, or by an A2A agonist. M1 macrophages incubated with NETs derived from patients with DADA2 released significantly greater amounts of TNF-α. Treatment with an A2AAR agonist decreased nuclear translocation of NF-κB and subsequent production of inflammatory cytokines in DADA2 monocyte-derived macrophages. These results suggest that neutrophils may play a pathogenic role in DADA2. Modulation of adenosine-mediated NET formation may contribute a novel and directed therapeutic approach in the treatment of DADA2 and potentially other inflammatory diseases.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



Comment in
-
NETing the mechanism of inflammation in DADA2.Blood. 2019 Jul 25;134(4):338-339. doi: 10.1182/blood.2019001251. Blood. 2019. PMID: 31345927 No abstract available.
References
-
- Ontyd J, Schrader J. Measurement of adenosine, inosine, and hypoxanthine in human plasma. J Chromatogr A. 1984;307(2):404-409. - PubMed
-
- Ramakers BP, Pickkers P, Deussen A, et al. . Measurement of the endogenous adenosine concentration in humans in vivo: methodological considerations. Curr Drug Metab. 2008;9(8):679-685. - PubMed
-
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64(4):785-795. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

