Proteostasis Environment Shapes Higher-Order Epistasis Operating on Antibiotic Resistance
- PMID: 31015194
- PMCID: PMC6553834
- DOI: 10.1534/genetics.119.302138
Proteostasis Environment Shapes Higher-Order Epistasis Operating on Antibiotic Resistance
Abstract
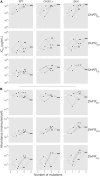
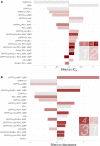
Recent studies have affirmed that higher-order epistasis is ubiquitous and can have large effects on complex traits. Yet, we lack frameworks for understanding how epistatic interactions are influenced by central features of cell physiology. In this study, we assess how protein quality control machinery-a critical component of cell physiology-affects epistasis for different traits related to bacterial resistance to antibiotics. Specifically, we disentangle the interactions between different protein quality control genetic backgrounds and two sets of mutations: (i) SNPs associated with resistance to antibiotics in an essential bacterial enzyme (dihydrofolate reductase, or DHFR) and (ii) differing DHFR bacterial species-specific amino acid background sequences (Escherichia coli, Listeria grayi, and Chlamydia muridarum). In doing so, we improve on generic observations that epistasis is widespread by discussing how patterns of epistasis can be partly explained by specific interactions between mutations in an essential enzyme and genes associated with the proteostasis environment. These findings speak to the role of environmental and genotypic context in modulating higher-order epistasis, with direct implications for evolutionary theory, genetic modification technology, and efforts to manage antimicrobial resistance.
Keywords: antibiotic resistance; epistasis; proteostasis.
Copyright © 2019 Guerrero et al.
Figures


Similar articles
-
Biophysical principles predict fitness landscapes of drug resistance.Proc Natl Acad Sci U S A. 2016 Mar 15;113(11):E1470-8. doi: 10.1073/pnas.1601441113. Epub 2016 Feb 29. Proc Natl Acad Sci U S A. 2016. PMID: 26929328 Free PMC article.
-
Epistasis and pleiotropy shape biophysical protein subspaces associated with drug resistance.Phys Rev E. 2023 Nov;108(5-1):054408. doi: 10.1103/PhysRevE.108.054408. Phys Rev E. 2023. PMID: 38115433 Free PMC article.
-
High-Order Epistasis in Catalytic Power of Dihydrofolate Reductase Gives Rise to a Rugged Fitness Landscape in the Presence of Trimethoprim Selection.Mol Biol Evol. 2019 Jul 1;36(7):1533-1550. doi: 10.1093/molbev/msz086. Mol Biol Evol. 2019. PMID: 30982891 Free PMC article.
-
Evolutionary Mechanisms Shaping the Maintenance of Antibiotic Resistance.Trends Microbiol. 2018 Aug;26(8):677-691. doi: 10.1016/j.tim.2018.01.005. Epub 2018 Feb 10. Trends Microbiol. 2018. PMID: 29439838 Review.
-
Epistasis and the Evolution of Antimicrobial Resistance.Front Microbiol. 2017 Feb 17;8:246. doi: 10.3389/fmicb.2017.00246. eCollection 2017. Front Microbiol. 2017. PMID: 28261193 Free PMC article. Review.
Cited by
-
Altered expression of a quality control protease in E. coli reshapes the in vivo mutational landscape of a model enzyme.Elife. 2020 Jul 23;9:e53476. doi: 10.7554/eLife.53476. Elife. 2020. PMID: 32701056 Free PMC article.
-
Learning high-order interactions for polygenic risk prediction.PLoS One. 2023 Feb 10;18(2):e0281618. doi: 10.1371/journal.pone.0281618. eCollection 2023. PLoS One. 2023. PMID: 36763605 Free PMC article.
-
Evolutionary "Crowdsourcing": Alignment of Fitness Landscapes Allows for Cross-species Adaptation of a Horizontally Transferred Gene.Mol Biol Evol. 2023 Nov 3;40(11):msad237. doi: 10.1093/molbev/msad237. Mol Biol Evol. 2023. PMID: 37931146 Free PMC article.
-
Epistasis decreases with increasing antibiotic pressure but not temperature.Philos Trans R Soc Lond B Biol Sci. 2023 May 22;378(1877):20220058. doi: 10.1098/rstb.2022.0058. Epub 2023 Apr 3. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37004727 Free PMC article.
-
Protein Quality Control is a Master Modulator of Molecular Evolution in Bacteria.Genome Biol Evol. 2025 Feb 3;17(2):evaf010. doi: 10.1093/gbe/evaf010. Genome Biol Evol. 2025. PMID: 39837347 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

